What volume would be occupied by a piece of aluminum (density = 2.70 g/mL) weighing 98.0 g?
A) 265 mL
B) 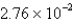 mL
mL
C) 36.3 mL
D) 3.63 mL
E) none of these
Correct Answer:
Verified
Q97: Convert: -12.2°C = _ °F.
A) -54.0°F
B) 10.0°F
C)
Q98: Convert: 21.9°C = _ °F.
A) 7.4°F
B) 44.2°F
C)
Q99: What is the Celsius equivalent of 436.5
Q100: Convert: -47.9°C = _ °F.
A) -118.2°F
B) 5.4°F
C)
Q101: Baking soda and vinegar are mixed in
Q103: A chemist needs 18.3 g of bromine
Q104: The density of an object that has
Q105: Perform the following conversion: 5.39 m/s =
Q106: A chunk of sulfur has a volume
Q107: A solid object with a volume of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents