A piece of an unknown metal weighs 400.1 g and occupies a volume of 72.2 mL. What is the density of this metal?
A) 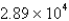 g/mL
g/mL
B) 5.54 g/mL
C) 0.180 g/mL
D) 55.4 g/mL
E) none of these
Correct Answer:
Verified
Q111: A graduated cylinder contains 20.0 mL of
Q112: An object is 153.8 inches in height.
Q113: Copper has a density of 8.96 g/cm3.
Q114: The density of gold is 19.3 g/mL.
Q115: Perform the following conversion: 6.41 m/s =
Q116: The density of copper is 8.92 g/mL.
Q117: A toy measures 39.1 cm in length.
Q119: An object has a mass of 40.1
Q120: How many cups are in a 64-oz
Q121: How many quarts are in a 11.6-gal
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents