Examine this reaction coordinate diagram, and answer the questions that follow. 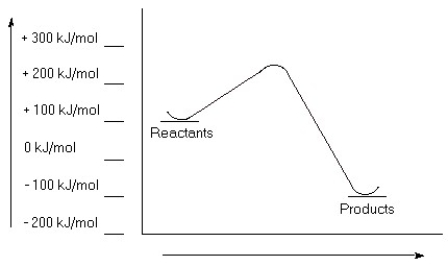 a) Estimate the value for the activation energy for this reaction.
a) Estimate the value for the activation energy for this reaction.
b) Calculate ΔErxn.
c) Is this reaction exothermic or endothermic?
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q77: If the forward reaction is exothermic, the
Q78: The magnitude (size) of the energy of
Q79: An exothermic reaction always leads to a
Q80: A lower energy of activation leads to
Q81: The reactants must collide with an energy
Q83: The greater the concentration of the reactants,
Q84: Based on the collision theory, decreasing temperature
Q85: Write the overall balanced equation that is
Q86: Compare the roles of catalysts and enzymes
Q87: The rate determining step is always the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents