Consider the reaction of A being converted into B at 25°C. If the ΔG° of this reaction is 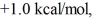 the Keq is ________ and the % conversion is ________.
the Keq is ________ and the % conversion is ________.
A) 0.18, 15%
B) 0.43, 30%
C) 1.0, 50%
D) 2.3, 70%
E) 5.4, 84%
Correct Answer:
Verified
Q20: If ΔG° for a given reaction at
Q21: Given a K of 0.45 at 25°C,
Q23: Given a K of 2.2 at 25°C,
Q24: Consider the reaction of A being converted
Q24: Consider the reaction of A being converted
Q25: Which is a measure of the randomness
Q27: For the reaction A + B →
Q29: Which of the following is true for
Q30: Provided the following pKa values for the
Q35: If the equilibrium constant (Keq) of a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents