Given the bond dissociation energies below (in kcal/mol) , estimate the ΔH° for the propagation step 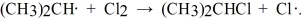
CH3CH2CH2-H 98
(CH3) 2CH-H 95
Cl-Cl 58
H-Cl 103
CH3CH2CH2-Cl 81
(CH3) 2CH-Cl 80
A) -22 kcal/mol
B) +22 kcal/mol
C) -40 kcal/mol
D) +45 kcal/mol
Correct Answer:
Verified
Q46: The bond dissociation energy is the amount
Q48: Given the bond dissociation energies below (in
Q49: Which compound has the smaller bond dissociation
Q53: Given the chlorination of acetone shown below,
Q56: Predict the signs of DH° and DS°
Q58: Of the two C-H bonds shown, which
Q58: Do you expect the initiation step in
Q59: The hydrogenation of acetylene to produce ethane
Q61: Explain the significance of the exponential factor
Q67: The difference in energy between reactants and
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents