Consider the conversion of X to Z through the sole intermediate Y. Given the reaction-energy diagram shown below, which step is the rate-limiting step? Explain your reasoning. 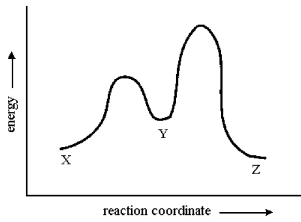
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q63: Explain the significance of the frequency factor
Q64: Consider the three-step mechanism for the reaction
Q65: In the hydrocarbon shown below, how many
Q67: The difference in energy between reactants and
Q68: Which of the following correctly expresses the
Q70: Consider the one-step conversion of F to
Q71: Consider the reaction: CH3CH2∙ + Br2 →
Q72: Consider the reaction (CH3)3CBr + CH3CH2OH →
Q78: Consider the conversion of C to D
Q78: Consider the three-step mechanism for the reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents