Remove an H+ from the following structure to create the most reactive (least stable) carbanion. 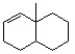
Correct Answer:
Verified
Q103: The hydrogen atom abstraction step in the
Q104: When Br radical reacts with 1-butene (CH3CH2CH=CH2),
Q119: When 1,1,3,3-tetramethylcyclobutane is brominated at 125°C, the
Q122: What reactive intermediate is formed when CHBr3
Q123: Describe the hybridization of the cationic center
Q124: What is the hybridization of the positively
Q125: When acetaldehyde (CH3CHO) is deprotonated, the resulting
Q126: What is the CCC bond angle in
Q126: What is the CCC bond angle in
Q128: What is the hybridization of the negatively
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents