Which of these pairs are geometrical isomers?
A) 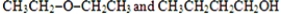
B) 
C) 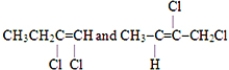
D) 
Correct Answer:
Verified
Q68: Which has resonance structures?
A) CH4
B) CH3CH2COO−
C) CH3CH2CH3
D)
Q72: What is the molecular formula for the
Q73: Which compound,if any,is optically active?
A) 
Q74: The two molecules represented below are examples
Q75: Which compound,if any,will not be optically active?
A)
Q76: What is the molecular formula for the
Q78: The two molecules represented below are examples
Q79: What is the name of the following
Q86: One characteristic of the monomers that form
Q99: Which of the following polymers is a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents