The following electrolytic cell is a representation of the process used to purify copper metal.How does it work? 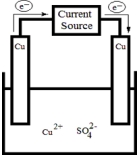 .
.
A) Impure copper is oxidized at the anode,and pure copper is reduced at the cathode.Impurities dissolve into solution.
B) Impure copper is oxidized at the cathode,and pure copper is reduced at the anode.Impurities dissolve into solution.
C) Impure copper is oxidized at the anode,and pure copper dissolves into solution.Impurities remain on the cathode.
D) Impure copper is oxidized at the cathode,and pure copper dissolves into solution.Impurities remain on the anode.
E) Impure copper precipitates from solution onto the cathode,and pure copper remains on the anode.
Correct Answer:
Verified
Q61: What is the chemical formula for talc?
A)
Q62: What type of metal is generally separated
Q66: Which diagram best corresponds to the energy
Q67: What is the chemical formula for limestone?
A)
Q73: A fractional distillation method called the Mond
Q74: What is the name of the process
Q74: Which diagram best corresponds to the energy
Q77: Which mineral is a silicate?
A) Cinnabar
B) Corundum
C)
Q78: Which ion is not found in sea
Q79: What is the chemical formula for cinnabar?
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents