Calculate the energy released in joules when one mole of polonium-214 decays according to the following equation. 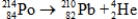 Particle Mass (amu)
Particle Mass (amu)
Pb-210
209) 98284
Po-214
213) 99519
He-4
4) 00260
(1 kg = 6.022 × 1026 amu;
NA = 6.022 × 1023 mol-1;
C = 2.99792458 × 108 m/s)
A) 8.78 × 1014 J/mol
B) 7.2 × 1014 J/mol
C) 8.76 × 1011 J/mol
D) -9.75 × 10-3 J/mol
E) 1.46 × 10-9 J/mol
Correct Answer:
Verified
Q21: What fraction of radioactive atoms remains in
Q22: 1 joule equals
A) 1 kg m
B) 1
Q22: The radiochemist, Will I. Glow, studied thorium-232
Q27: Iodine-131, t1/2 = 8.0 days, is used
Q29: What is the nuclear binding energy per
Q32: Cesium-134 is a β emitter with a
Q34: The nuclear binding energy per nucleon is
Q34: What is the name for the difference
Q36: The 14C activity of some ancient Peruvian
Q48: Charcoal found under a stone at Stonehenge,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents