
The Living World 8th Edition by George Johnson
النسخة 8الرقم المعياري الدولي: 978-0078024214
The Living World 8th Edition by George Johnson
النسخة 8الرقم المعياري الدولي: 978-0078024214 تمرين 1
Using Radioactive Decay to Date the Iceman
In the fall of 1991, sticking out of the melting snow on the crest of a high pass near the mountainous border between Italy and Austria, two Austrian hikers found a corpse. Right away it was clear the body was very old, frozen in an icy trench where he had sought shelter long ago and only now released as the ice melted. In the years since this startling find, scientists have learned a great deal about the dead man, whom they named Ötzi. They know his age, his health, the shoes and clothing he wore, what he ate, and that he died from an arrow that ripped through his back. Its tip is still embedded in the back of his left shoulder. From the distribution of chemicals in his teeth and bones, we know he lived his life within 60 kilometers of where he died.
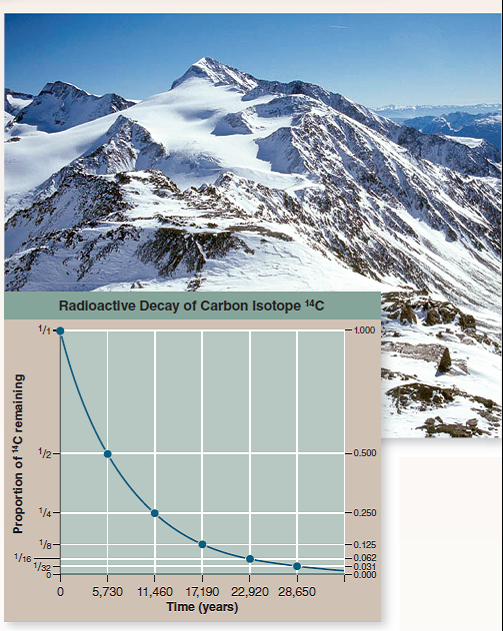
How long ago did this iceman die? Scientists answered this key question by measuring the degree of decay of the short-lived carbon isotope 14 C in Ötzi's body. This procedure is discussed earlier in this chapter (see figure 2.8). The graph to the right displays the radioactive decay curve of the carbon isotope carbon-14 ( 14 C); it takes 5,730 years for half of the 14 C present in a sample to decay to nitrogen-14 ( 14 N). When Ötzi's carbon isotopes were analyzed, researchers determined that the ratio of 14 C to 12 C (a ratio is the size of one variable relative to another), also written as the fraction 14 C/ 12 C, in Ötzi's body is 0.435 of the fraction found in tissues of a person who has recently died.
Further Analysis
a. The radioactive iodine isotope 131 I decays at a half-life of eight days. Plotted on the graph above, would its radioactive decay curve be above or below that of 14 C?
b. scientists often employ the radioactive decay of isotope potassium-40 ( 40 K) into argon-40 ( 40 Ar) to date old material. 40 K has a half-life of 1.3 billion years. Would it be a better isotope than 14 C for dating Ötzi?
Figure 2.8 Radioactive isotope dating.
This diagram illustrates radioactive dating using carbon-14, a short-lived isotope.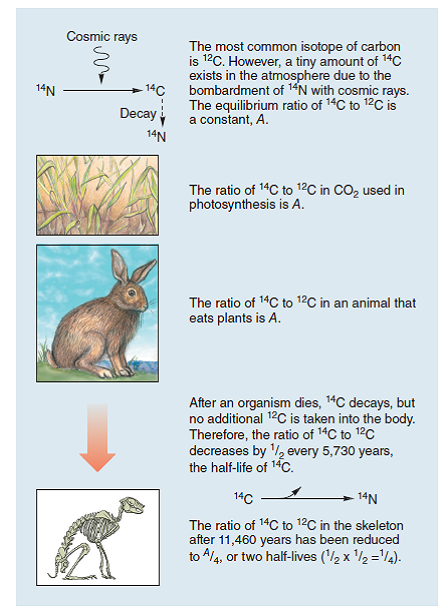
In the fall of 1991, sticking out of the melting snow on the crest of a high pass near the mountainous border between Italy and Austria, two Austrian hikers found a corpse. Right away it was clear the body was very old, frozen in an icy trench where he had sought shelter long ago and only now released as the ice melted. In the years since this startling find, scientists have learned a great deal about the dead man, whom they named Ötzi. They know his age, his health, the shoes and clothing he wore, what he ate, and that he died from an arrow that ripped through his back. Its tip is still embedded in the back of his left shoulder. From the distribution of chemicals in his teeth and bones, we know he lived his life within 60 kilometers of where he died.
How long ago did this iceman die? Scientists answered this key question by measuring the degree of decay of the short-lived carbon isotope 14 C in Ötzi's body. This procedure is discussed earlier in this chapter (see figure 2.8). The graph to the right displays the radioactive decay curve of the carbon isotope carbon-14 ( 14 C); it takes 5,730 years for half of the 14 C present in a sample to decay to nitrogen-14 ( 14 N). When Ötzi's carbon isotopes were analyzed, researchers determined that the ratio of 14 C to 12 C (a ratio is the size of one variable relative to another), also written as the fraction 14 C/ 12 C, in Ötzi's body is 0.435 of the fraction found in tissues of a person who has recently died.


Further Analysis
a. The radioactive iodine isotope 131 I decays at a half-life of eight days. Plotted on the graph above, would its radioactive decay curve be above or below that of 14 C?
b. scientists often employ the radioactive decay of isotope potassium-40 ( 40 K) into argon-40 ( 40 Ar) to date old material. 40 K has a half-life of 1.3 billion years. Would it be a better isotope than 14 C for dating Ötzi?
Figure 2.8 Radioactive isotope dating.
This diagram illustrates radioactive dating using carbon-14, a short-lived isotope.

التوضيح
In this question, we consider two questi...
The Living World 8th Edition by George Johnson
لماذا لم يعجبك هذا التمرين؟
أخرى 8 أحرف كحد أدنى و 255 حرفاً كحد أقصى
حرف 255








