
The Living World 8th Edition by George Johnson
النسخة 8الرقم المعياري الدولي: 978-0078024214
The Living World 8th Edition by George Johnson
النسخة 8الرقم المعياري الدولي: 978-0078024214 تمرين 9
How Do Swimming Fish Avoid Low Blood pH?
Animals that live in oxygen-poor environments, like worms living in the oxygen-free mud at the bottom of lakes, are not able to obtain the energy required for muscle movement from the Krebs cycle. Their cells lack the oxygen needed to accept the electrons stripped from food molecules. Instead, these animals rely on glycolysis to obtain ATP, donating the electron to pyruvate, forming lactic acid. While much less efficient than the Krebs cycle, glycolysis does not require oxygen. Even when oxygen is plentiful, the muscles of an active animal may use up oxygen more quickly than it can be supplied by the bloodstream and so be forced to temporarily rely on glycolysis to generate the ATP for continued contraction.
This presents a particular problem for fish. Fish blood is much lower in carbon dioxide than yours is, and as a consequence, the amount of sodium bicarbonate acting as a buffer in fish blood is also quite low. Now imagine you are a trout and need to suddenly swim very fast to catch a mayfly for dinner. The vigorous swimming will cause your muscles to release large amounts of lactic acid into your poorly buffered blood; this could severely disturb the blood's acid-base balance and so impede contraction of your swimming muscles before the prey is captured.
The graph to the right presents the results of an experiment designed to explore how a trout solves this dilemma. In the experiment, the trout was made to swim vigorously for 15 minutes in a laboratory tank and then allowed a day's recovery. The lactic acid concentration in its blood was monitored periodically during swimming and recovery phases.
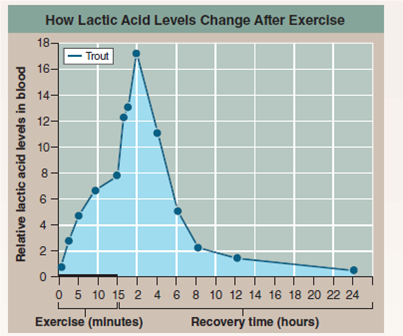
Drawing Conclusions Is this result consistent with the hypothesis that fish maintain blood pH levels by delaying the release of lactic acid from muscles? Why might this be beneficial to the fish?
Animals that live in oxygen-poor environments, like worms living in the oxygen-free mud at the bottom of lakes, are not able to obtain the energy required for muscle movement from the Krebs cycle. Their cells lack the oxygen needed to accept the electrons stripped from food molecules. Instead, these animals rely on glycolysis to obtain ATP, donating the electron to pyruvate, forming lactic acid. While much less efficient than the Krebs cycle, glycolysis does not require oxygen. Even when oxygen is plentiful, the muscles of an active animal may use up oxygen more quickly than it can be supplied by the bloodstream and so be forced to temporarily rely on glycolysis to generate the ATP for continued contraction.
This presents a particular problem for fish. Fish blood is much lower in carbon dioxide than yours is, and as a consequence, the amount of sodium bicarbonate acting as a buffer in fish blood is also quite low. Now imagine you are a trout and need to suddenly swim very fast to catch a mayfly for dinner. The vigorous swimming will cause your muscles to release large amounts of lactic acid into your poorly buffered blood; this could severely disturb the blood's acid-base balance and so impede contraction of your swimming muscles before the prey is captured.
The graph to the right presents the results of an experiment designed to explore how a trout solves this dilemma. In the experiment, the trout was made to swim vigorously for 15 minutes in a laboratory tank and then allowed a day's recovery. The lactic acid concentration in its blood was monitored periodically during swimming and recovery phases.


Drawing Conclusions Is this result consistent with the hypothesis that fish maintain blood pH levels by delaying the release of lactic acid from muscles? Why might this be beneficial to the fish?
التوضيح
The research suggests that severe exerci...
The Living World 8th Edition by George Johnson
لماذا لم يعجبك هذا التمرين؟
أخرى 8 أحرف كحد أدنى و 255 حرفاً كحد أقصى
حرف 255








