
Becker's World of the Cell 9th Edition by Lewis Kleinsmith, Jeff Hardin, Gregory Paul Bertoni
النسخة 9الرقم المعياري الدولي: 9780134295510
Becker's World of the Cell 9th Edition by Lewis Kleinsmith, Jeff Hardin, Gregory Paul Bertoni
النسخة 9الرقم المعياري الدولي: 9780134295510 تمرين 8
QUANTITATIVE Bond Energies. A single covalent bond has a bond energy of approximately 90 kcal/mol, and a typical hydrogen bond has a bond energy of about 5 kcal/mol. Although weak, hydrogen bonds can be a major structural force when present in large numbers as in DNA. In double-stranded DNA, each AT base pair is held together by two hydrogen bonds, and each GC base pair is held together by three hydrogen bonds.
(a)What is the total bond energy in a propane molecule (see Figure)The C - C bond energy is 83 kcal/mol, and the C-H bond energy is 99 kcal/mol.
(b)In a short 15 base-pair molecule of DNA having 60% GC pairs and 40% AT pairs, what is the total bond energy of all the hydrogen bonds How does this compare to the bond energy of a carbon-carbon bond
(c)In a typical gene consisting of 1000 base pairs with the same relative GC versus AT content, what is the total bond energy of all the hydrogen bonds How does this compare to the bond energy of a carbon-carbon bond
Figure Some Simple Hydrocarbon Compounds. Compounds in the top row have single bonds only, whereas those in the second and third rows have double or triple bonds.
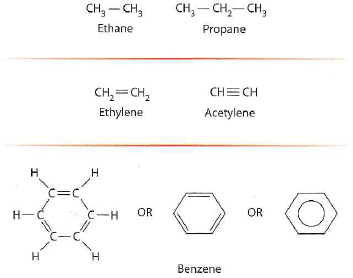
(a)What is the total bond energy in a propane molecule (see Figure)The C - C bond energy is 83 kcal/mol, and the C-H bond energy is 99 kcal/mol.
(b)In a short 15 base-pair molecule of DNA having 60% GC pairs and 40% AT pairs, what is the total bond energy of all the hydrogen bonds How does this compare to the bond energy of a carbon-carbon bond
(c)In a typical gene consisting of 1000 base pairs with the same relative GC versus AT content, what is the total bond energy of all the hydrogen bonds How does this compare to the bond energy of a carbon-carbon bond
Figure Some Simple Hydrocarbon Compounds. Compounds in the top row have single bonds only, whereas those in the second and third rows have double or triple bonds.

التوضيح
The bond energies for the different mole...
Becker's World of the Cell 9th Edition by Lewis Kleinsmith, Jeff Hardin, Gregory Paul Bertoni
لماذا لم يعجبك هذا التمرين؟
أخرى 8 أحرف كحد أدنى و 255 حرفاً كحد أقصى
حرف 255








