
Biomedical Engineering: Bridging Medicine and Technology 1st Edition by Veronique Tran,Mark Saltzman
النسخة 1الرقم المعياري الدولي: 9780521840996
Biomedical Engineering: Bridging Medicine and Technology 1st Edition by Veronique Tran,Mark Saltzman
النسخة 1الرقم المعياري الدولي: 9780521840996 تمرين 28
The decomposition of calcium carbonate is shown below along with the standard enthalpy and entropy values.
a. Calculate the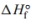 for the reaction.
for the reaction.
b. Calculate the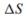 for the reaction.
for the reaction.
c. What is the standard Gibb's free-energy change expression for the reac- tion?
d. Is the reaction spontaneous at 25◦C? Is the reaction spontaneous at 1000◦C? Explain your answers.
e. Calculate the equilibrium constant at 25◦C and 1000◦C.
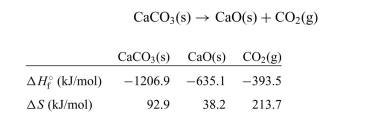
a. Calculate the
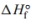 for the reaction.
for the reaction. b. Calculate the
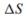 for the reaction.
for the reaction. c. What is the standard Gibb's free-energy change expression for the reac- tion?
d. Is the reaction spontaneous at 25◦C? Is the reaction spontaneous at 1000◦C? Explain your answers.
e. Calculate the equilibrium constant at 25◦C and 1000◦C.

التوضيح
a) The ?H o f for the reaction is -2235....
Biomedical Engineering: Bridging Medicine and Technology 1st Edition by Veronique Tran,Mark Saltzman
لماذا لم يعجبك هذا التمرين؟
أخرى 8 أحرف كحد أدنى و 255 حرفاً كحد أقصى
حرف 255








