
Biomedical Engineering: Bridging Medicine and Technology 1st Edition by Veronique Tran,Mark Saltzman
النسخة 1الرقم المعياري الدولي: 9780521840996
Biomedical Engineering: Bridging Medicine and Technology 1st Edition by Veronique Tran,Mark Saltzman
النسخة 1الرقم المعياري الدولي: 9780521840996 تمرين 30
The reaction in which urea is formed from NH3 and CO2 is shown below. The standard free-energy change 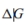 at 25◦Cis−13.6 kJ/mol.
at 25◦Cis−13.6 kJ/mol. 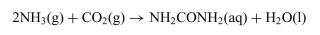
a. Write an expression for the equilibrium constant, K, in terms of the molar concentrations of the reactants and products.
b. Write an expression for the equilibrium constant, K, as a function of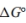 and temperature.
and temperature.
c. Determine the value of the equilibrium constant, K, for this reaction at 25◦C.
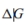 at 25◦Cis−13.6 kJ/mol.
at 25◦Cis−13.6 kJ/mol. 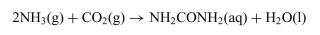
a. Write an expression for the equilibrium constant, K, in terms of the molar concentrations of the reactants and products.
b. Write an expression for the equilibrium constant, K, as a function of
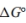 and temperature.
and temperature. c. Determine the value of the equilibrium constant, K, for this reaction at 25◦C.
التوضيح
a) Expression for the equilibrium consta...
Biomedical Engineering: Bridging Medicine and Technology 1st Edition by Veronique Tran,Mark Saltzman
لماذا لم يعجبك هذا التمرين؟
أخرى 8 أحرف كحد أدنى و 255 حرفاً كحد أقصى
حرف 255








