Deck 22: Organic Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/51
Play
Full screen (f)
Deck 22: Organic Chemistry
1
What is the IUPAC name of the following compound? 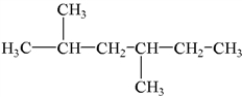
A) 2,4-dimethylhexane
B) 2-ethyl-4-methylpentane
C) 2,4-methylhexane
D) 3,5-dimethylhexane
E) 2,4-octane

A) 2,4-dimethylhexane
B) 2-ethyl-4-methylpentane
C) 2,4-methylhexane
D) 3,5-dimethylhexane
E) 2,4-octane
2,4-dimethylhexane
2
Cylinders of bottled gas used in campstoves and grills contain liquid
A) methane.
B) ethane.
C) propane
D) acetylene.
E) ethene.
A) methane.
B) ethane.
C) propane
D) acetylene.
E) ethene.
propane
3
How many π bonds are present in (the noncyclic hydrocarbon)C3H4?
A) 0
B) 1
C) 2
D) 3
E) 6
A) 0
B) 1
C) 2
D) 3
E) 6
2
4
What are the approximate bond angles in C3H8?
A) 60°
B) 90°
C) 109.5°
D) 120°
E) 90° and 120°
A) 60°
B) 90°
C) 109.5°
D) 120°
E) 90° and 120°

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
5
The octane number of a gasoline is a measure of
A) the mass of octane in a 100 gram sample of gasoline.
B) its resistance to knock (i.e. ,premature ignition).
C) the mass of a gasoline sample divided by the molar mass of octane.
D) the vapor pressure of gasoline at 298 K.
E) the percentage of octane in a sample of gasoline.
A) the mass of octane in a 100 gram sample of gasoline.
B) its resistance to knock (i.e. ,premature ignition).
C) the mass of a gasoline sample divided by the molar mass of octane.
D) the vapor pressure of gasoline at 298 K.
E) the percentage of octane in a sample of gasoline.

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
6
What is the name of the following compound? 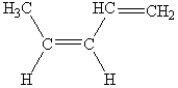
A) trans-acetylene
B) cis-1,3-pentadiene
C) trans-2,4-pentadiene
D) cis-4-methyl-1,3-butadiene
E) cis-4-methyl-1,3-butene

A) trans-acetylene
B) cis-1,3-pentadiene
C) trans-2,4-pentadiene
D) cis-4-methyl-1,3-butadiene
E) cis-4-methyl-1,3-butene

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
7
What type of intermolecular forces or bonds dominate in alkanes?
A) Dispersion forces
B) Dipole forces
C) Hydrogen bonds
D) Covalent bonds
E) Ionic bonds
A) Dispersion forces
B) Dipole forces
C) Hydrogen bonds
D) Covalent bonds
E) Ionic bonds

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following alkanes will have the lowest boiling point?
A)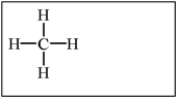
B)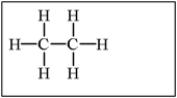
C)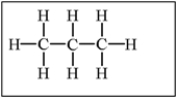
D)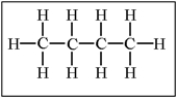
E) All the above alkanes have the same boiling point.
A)

B)

C)

D)

E) All the above alkanes have the same boiling point.

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following (non-cyclic)molecules is an alkyne?
A) CH4
B) C2H2
C) C2H6
D) C3H6
E) C6H14
A) CH4
B) C2H2
C) C2H6
D) C3H6
E) C6H14

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
10
What is the name of the following compound? 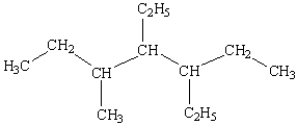
A) 3,4-diethyl-5-methylheptane
B) 3,4,5-heptane
C) 3-ethyl-5-(2-butyl)hexane
D) 3-(2-butyl)-3-ethylhexane
E) 3,5,6-trimethylheptane

A) 3,4-diethyl-5-methylheptane
B) 3,4,5-heptane
C) 3-ethyl-5-(2-butyl)hexane
D) 3-(2-butyl)-3-ethylhexane
E) 3,5,6-trimethylheptane

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
11
What is the hybridization of the carbon atoms in an alkane?
A) σ
B) π
C) sp
D) sp2
E) sp3
A) σ
B) π
C) sp
D) sp2
E) sp3

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
12
What is the IUPAC name of the following compound? 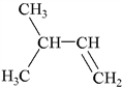
A) 3-methyl-1-butyne
B) 3-methyl-1-butene
C) 2-methyl-3-butene
D) 2-methyl-4-butene
E) 3,3-dimethyl-1-propene

A) 3-methyl-1-butyne
B) 3-methyl-1-butene
C) 2-methyl-3-butene
D) 2-methyl-4-butene
E) 3,3-dimethyl-1-propene

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
13
Gasoline is
A) 90% isooctane and 10% antiknocking compounds.
B) primarily composed of methyl-t-butyl ether (MTBE).
C) primarily composed of isooctane.
D) a mixture of hydrocarbons in the C5 to C12 range.
E) more volatile than propane,but less volatile than butane.
A) 90% isooctane and 10% antiknocking compounds.
B) primarily composed of methyl-t-butyl ether (MTBE).
C) primarily composed of isooctane.
D) a mixture of hydrocarbons in the C5 to C12 range.
E) more volatile than propane,but less volatile than butane.

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
14
How many structural isomers exist for C5H12?
A) 2
B) 3
C) 4
D) 5
E) 6
A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
15
What is the name of the following compound? 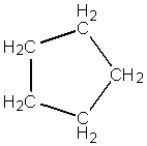
A) pentanol
B) cyclopentane
C) cyclohexane
D) cyclopentene
E) 1,5-pentane

A) pentanol
B) cyclopentane
C) cyclohexane
D) cyclopentene
E) 1,5-pentane

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following alkanes will have the highest boiling point?
A)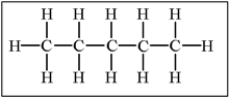
B)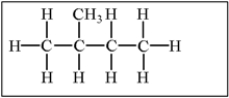
C)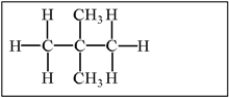
D)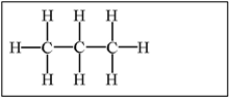
E) Molecules a,b,and c have equally high boiling points.
A)

B)

C)

D)

E) Molecules a,b,and c have equally high boiling points.

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
17
What process is used to separate petroleum into various products,such as gasoline,kerosene,and fuel oil?
A) Mass spectroscopy
B) Filtration
C) Gas chromatography
D) Column chromatography
E) Fractional distillation
A) Mass spectroscopy
B) Filtration
C) Gas chromatography
D) Column chromatography
E) Fractional distillation

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
18
What is the hybridization of each carbon atom in acetylene,C2H2?
A) sp
B) sp2
C) sp3
D) sp3d
E) 2σ and 2π
A) sp
B) sp2
C) sp3
D) sp3d
E) 2σ and 2π

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
19
What is the general formula for an alkane?
A) CnH2n+2
B) CnH2n
C) CnHn-2
D) Cn+2Hn
E) CnH2n-2
A) CnH2n+2
B) CnH2n
C) CnHn-2
D) Cn+2Hn
E) CnH2n-2

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
20
All of the following statements concerning ethene are true EXCEPT
A) ethene is the most produced organic compound in the United States.
B) ethene is the starting material for polyethylene.
C) small quantities of ethene are used to ripen fruit.
D) ethene is a chiral molecule.
E) ethene has the molecular formula C2H4.
A) ethene is the most produced organic compound in the United States.
B) ethene is the starting material for polyethylene.
C) small quantities of ethene are used to ripen fruit.
D) ethene is a chiral molecule.
E) ethene has the molecular formula C2H4.

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
21
What is the IUPAC name of the following compound? 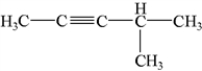
A) 4-methyl-2-pentyne
B) 4-methyl-2,3-pentyne
C) 4-methyl-2,3-dipentyne
D) 2-methyl-3-pentyne
E) 2-methyl-3,4-pentyne
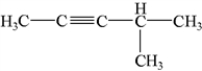
A) 4-methyl-2-pentyne
B) 4-methyl-2,3-pentyne
C) 4-methyl-2,3-dipentyne
D) 2-methyl-3-pentyne
E) 2-methyl-3,4-pentyne

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
22
Formulas for derivatives of hydrocarbons may be written as R−X,where R is a hydrocarbon lacking a hydrogen atom and X is a functional group.Which of the following formulas represents a ketone?
A) ROH
B) ROR'
C) RCOR'
D) RCO2R'
E) RCHO
A) ROH
B) ROR'
C) RCOR'
D) RCO2R'
E) RCHO

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
23
How many different structures (including structural and geometric isomers)exist for C2H2Cl2?
A) 2
B) 3
C) 4
D) 5
E) 6
A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
24
What is the hybridization of each carbon atom in benzene,C6H6?
A) 2σ
B) 2π
C) sp
D) sp2
E) sp3
A) 2σ
B) 2π
C) sp
D) sp2
E) sp3

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
25
Classify the following molecule according to its functional group. 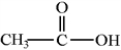
A) alcohol
B) aldehyde
C) alkane
D) carboxylic acid
E) ester
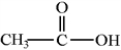
A) alcohol
B) aldehyde
C) alkane
D) carboxylic acid
E) ester

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
26
Formulas for derivatives of hydrocarbons may be written as R−X,where R is a hydrocarbon lacking a hydrogen atom and X is a functional group.Which of the following formulas represents an ester?
A) ROH
B) ROR'
C) RCOR'
D) RCO2R'
E) RCHO
A) ROH
B) ROR'
C) RCOR'
D) RCO2R'
E) RCHO

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
27
Classify the following molecule according to its functional group. 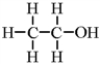
A) alcohol
B) aldehyde
C) carboxylic acid
D) ester
E) ether
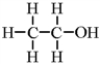
A) alcohol
B) aldehyde
C) carboxylic acid
D) ester
E) ether

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
28
Which compounds often have strong,pleasant odors?
A) alcohols
B) aldehydes
C) amines
D) esters
E) ethers
A) alcohols
B) aldehydes
C) amines
D) esters
E) ethers

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
29
How many isomers exist for the following benzene derivative,C6H4ClBr?
A) 2
B) 3
C) 4
D) 5
E) none
A) 2
B) 3
C) 4
D) 5
E) none

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
30
How many different (non-cyclic)structural isomers exist for C6H12?
A) 4
B) 8
C) 10
D) 12
E) 13
A) 4
B) 8
C) 10
D) 12
E) 13

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
31
Classify the following molecule according to its functional group. 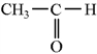
A) alcohol
B) aldehyde
C) carboxylic acid
D) ether
E) ketone
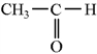
A) alcohol
B) aldehyde
C) carboxylic acid
D) ether
E) ketone

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
32
Which functional group does not contain a double bond to an oxygen atom?
A) ester
B) aldehyde
C) ether
D) amide
E) ketone
A) ester
B) aldehyde
C) ether
D) amide
E) ketone

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
33
What is the name of the following benzene derivative? 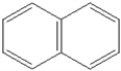
A) dibenzene
B) 1,2-dibenzene
C) toluene
D) naphthalene
E) aniline

A) dibenzene
B) 1,2-dibenzene
C) toluene
D) naphthalene
E) aniline

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
34
Classify the following molecule according to its functional group. 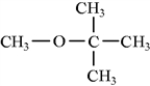
A) alcohol
B) carboxylic acid
C) ketone
D) ester
E) ether

A) alcohol
B) carboxylic acid
C) ketone
D) ester
E) ether

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
35
How many ketones have the chemical formula C5H10O?
A) 2
B) 3
C) 4
D) 5
E) 6
A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the structures below has the common name o-dichlorobenzene (where o- is ortho)?
A)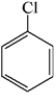
B)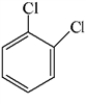
C)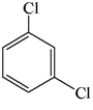
D)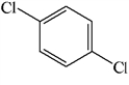
E)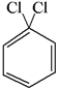
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
37
What is the name of the following benzene derivative? 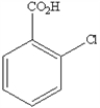
A) 1-chlorobenzoic acid
B) 5-chloroanaline
C) 1-acetate-1-chlorobenzene
D) 5-chlorobenzoic acid
E) 1,3-chlorocarboxylic benzene

A) 1-chlorobenzoic acid
B) 5-chloroanaline
C) 1-acetate-1-chlorobenzene
D) 5-chlorobenzoic acid
E) 1,3-chlorocarboxylic benzene

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
38
How many ethers have the chemical formula C5H12O?
A) 3
B) 4
C) 5
D) 6
E) 7
A) 3
B) 4
C) 5
D) 6
E) 7

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
39
How many alcohols have the chemical formula C4H10O?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
40
Which one of the following hydrocarbons is aromatic?
A)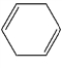
B)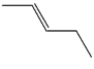
C)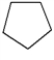
D)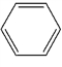
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following is an elimination reaction?
A) C2H2(g)+ H2(g)→ C2H4(g)
B) CH3CH2CH2OH(l)→ CH3CHCH2(g)+ H2O(l)
C) C3H6(g)+ HCl(g)→ C3H7Cl(l)
D) CH3OH(aq)+ HCOOH(aq)→ C2H5−O−CHO(l)+ H2O(l)
E) 2C2H2(g)+ 5O2(g)→ 4CO2(g)+ 2H2O(g)
A) C2H2(g)+ H2(g)→ C2H4(g)
B) CH3CH2CH2OH(l)→ CH3CHCH2(g)+ H2O(l)
C) C3H6(g)+ HCl(g)→ C3H7Cl(l)
D) CH3OH(aq)+ HCOOH(aq)→ C2H5−O−CHO(l)+ H2O(l)
E) 2C2H2(g)+ 5O2(g)→ 4CO2(g)+ 2H2O(g)

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following is a substitution reaction?
A) C2H2(g)+ H2(g)→ C2H4(g)
B) C6H6(l)+ HNO3(l)→ C6H5NO2(l)+ H2O(l)
C) CH3CH2CH2OH(l)→ CH3CHCH2(g)+ H2O(l)
D) CH3OH(aq)+ HCOOH(aq)→ C2H5−O−CHO(l)+ H2O(l)
E) 2C2H2(g)+ 5O2(g)→ 4CO2(g)+ 2H2O(g)
A) C2H2(g)+ H2(g)→ C2H4(g)
B) C6H6(l)+ HNO3(l)→ C6H5NO2(l)+ H2O(l)
C) CH3CH2CH2OH(l)→ CH3CHCH2(g)+ H2O(l)
D) CH3OH(aq)+ HCOOH(aq)→ C2H5−O−CHO(l)+ H2O(l)
E) 2C2H2(g)+ 5O2(g)→ 4CO2(g)+ 2H2O(g)

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following molecules has at least one chiral center? 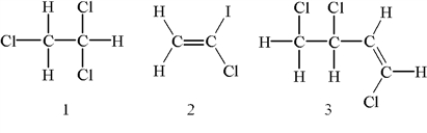
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 2 and 3

A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 2 and 3

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
44
For which one of the following molecules do geometric isomers exist?
A) H2ClC−CHCl2
B) BrHC=CHBr
C)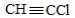
D) H3C−CH2Cl
E) H2C=CCl2
A) H2ClC−CHCl2
B) BrHC=CHBr
C)
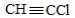
D) H3C−CH2Cl
E) H2C=CCl2

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
45
Alkenes undergo addition reactions with halogens.What is the product of the reaction of propylene,C3H6,with Br2?
A)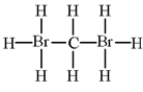
B)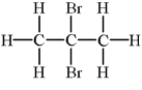
C)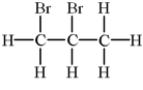
D)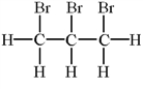
E)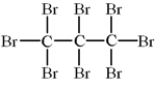
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
46
Write a balanced chemical equation for the reaction of ethene with oxygen.
A) CH4(g)+ 2O2(g)→ CO2(g)+ 2H2O(g)
B) 2C2H6(g)+ 7O2(g)→ 4CO2(g)+ 6H2O(g)
C) 2C2H2(g)+ 5O2(g)→ 4CO2(g)+ 2H2O(g)
D) C2H4(g)+ 3O2(g)→ 2CO2(g)+ 2H2O(g)
E) C2H4(g)+ 2O2(g)→ 2CO2(g)+ 2H2(g)
A) CH4(g)+ 2O2(g)→ CO2(g)+ 2H2O(g)
B) 2C2H6(g)+ 7O2(g)→ 4CO2(g)+ 6H2O(g)
C) 2C2H2(g)+ 5O2(g)→ 4CO2(g)+ 2H2O(g)
D) C2H4(g)+ 3O2(g)→ 2CO2(g)+ 2H2O(g)
E) C2H4(g)+ 2O2(g)→ 2CO2(g)+ 2H2(g)

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
47
Optical isomerism occurs when
A) a molecule's mirror image is superimposable.
B) both cis and trans isomers exist in equal concentrations for a particular molecule.
C) two or more structural isomers of a molecule exist.
D) at least one carbon atom in a molecule is bonded to four different atoms or groups.
E) both enantiomers are present in a racemic mixture.
A) a molecule's mirror image is superimposable.
B) both cis and trans isomers exist in equal concentrations for a particular molecule.
C) two or more structural isomers of a molecule exist.
D) at least one carbon atom in a molecule is bonded to four different atoms or groups.
E) both enantiomers are present in a racemic mixture.

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
48
What is the product of the addition of HCl to ethylene?
A) chloroethane
B) chloroethylene
C) 1,2-dichloroethane
D) 1,1-dichloroethane
E) 1,1,2,2-tetrachloroethylene
A) chloroethane
B) chloroethylene
C) 1,2-dichloroethane
D) 1,1-dichloroethane
E) 1,1,2,2-tetrachloroethylene

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
49
Which one of the following statements concerning isomers is INCORRECT?
A) Pairs of nonsuperimposable molecules are called enantiomers.
B) Enantiomers have identical melting and boiling points.
C) Molecules with two or more geometric isomers are termed chiral pairs.
D) Optical isomers rotate polarized light in opposite directions.
E) Structural isomers have the same composition,but the atoms are linked in different ways.
A) Pairs of nonsuperimposable molecules are called enantiomers.
B) Enantiomers have identical melting and boiling points.
C) Molecules with two or more geometric isomers are termed chiral pairs.
D) Optical isomers rotate polarized light in opposite directions.
E) Structural isomers have the same composition,but the atoms are linked in different ways.

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following molecules have chiral centers? 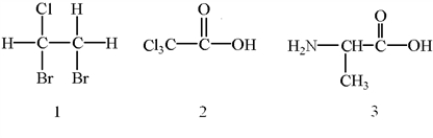
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1 and 3

A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1 and 3

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
51
The cholesterol molecule is found in two types of complexes: low-density lipoproteins (LDL)and high-density lipoproteins (HDL).Which of these is considered "bad"?
A) (LDL)
B) (HDL)
C) both (HDL)and (LDL),because they produce trans fat.
D) Neither,only omega-3 fatty acids are bad
E) both (HDL)and (LDL),because they form saturated fat.
A) (LDL)
B) (HDL)
C) both (HDL)and (LDL),because they produce trans fat.
D) Neither,only omega-3 fatty acids are bad
E) both (HDL)and (LDL),because they form saturated fat.

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck



