Deck 21: Electrochemistry Chemical Change and Electrical Work
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/100
Play
Full screen (f)
Deck 21: Electrochemistry Chemical Change and Electrical Work
1
When the following redox equation is balanced with smallest whole number coefficients, the coefficient for the hydrogen sulfate ion will be Al(s) + HSO4(aq) + OH-(aq) → Al2O3(s) + S2-(aq) + H2O(l)
A) 1.
B) 3.
C) 4.
D) 8.
E) None of these choices are correct.
A) 1.
B) 3.
C) 4.
D) 8.
E) None of these choices are correct.
3.
2
A voltaic cell is prepared using copper and silver. Its cell notation is shown below. Cu(s) | Cu2+(aq) || Ag+(aq) | Ag(s)
Which of the following processes occurs at the cathode?
A) Cu(s) → Cu2+(aq) + 2e-
B) Cu2+(aq) + 2e- → Cu(s)
C) Ag(s) → Ag+(aq) + e-
D) Ag+(aq) + e- → Ag(s)
E) Cu(s) + 2Ag+(aq) → Cu2+(aq) + 2Ag(s)
Which of the following processes occurs at the cathode?
A) Cu(s) → Cu2+(aq) + 2e-
B) Cu2+(aq) + 2e- → Cu(s)
C) Ag(s) → Ag+(aq) + e-
D) Ag+(aq) + e- → Ag(s)
E) Cu(s) + 2Ag+(aq) → Cu2+(aq) + 2Ag(s)
Ag+(aq) + e- → Ag(s)
3
When the following redox equation is balanced with smallest whole number coefficients, the coefficient for zinc will be Zn(s) + ReO4(aq) → Re(s) + Zn2+(aq) (acidic solution)
A) 2.
B) 7.
C) 8.
D) 16.
E) None of these choices are correct.
A) 2.
B) 7.
C) 8.
D) 16.
E) None of these choices are correct.
7.
4
Which of the following statements about voltaic and electrolytic cells is correct?
A) The anode will definitely gain weight in a voltaic cell.
B) Oxidation occurs at the cathode of both cells.
C) The free energy change, ΔG, is negative for the voltaic cell.
D) The electrons in the external wire flow from cathode to anode in an electrolytic cell.
E) None of these choices are correct.
A) The anode will definitely gain weight in a voltaic cell.
B) Oxidation occurs at the cathode of both cells.
C) The free energy change, ΔG, is negative for the voltaic cell.
D) The electrons in the external wire flow from cathode to anode in an electrolytic cell.
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
5
A voltaic cell prepared using aluminum and nickel has the following cell notation. Al(s) | Al3+(aq) || Ni2+(aq) | Ni(s)
Which of the following reactions occurs at the anode?
A) Al(s) → Al3+(aq) + 3e-
B) Al3+(aq) + 3e → Al(s)
C) Ni(s) → Ni2+(aq) + 2e-
D) Ni2+(aq) + 2e- → Ni(s)
E) None of these choices are correct.
Which of the following reactions occurs at the anode?
A) Al(s) → Al3+(aq) + 3e-
B) Al3+(aq) + 3e → Al(s)
C) Ni(s) → Ni2+(aq) + 2e-
D) Ni2+(aq) + 2e- → Ni(s)
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
6
A voltaic cell prepared using zinc and iodine has the following cell notation. Zn(s) | Zn2+(aq) || I-(aq) | I2(s) | C(graphite)
Which of the following equations correctly represents the balanced, spontaneous, cell reaction?
A) 2I-(aq) + Zn2+(aq) → I2(s) + Zn(s)
B) I2(s) + Zn(s) → 2I-(aq) + Zn2+(aq)
C) 2I-(aq) + Zn(s) → I2(s) + Zn2+(aq)
D) I2(s) + Zn2+(aq) → 2I-(aq) + Zn(s)
E) None of these choices are correct.
Which of the following equations correctly represents the balanced, spontaneous, cell reaction?
A) 2I-(aq) + Zn2+(aq) → I2(s) + Zn(s)
B) I2(s) + Zn(s) → 2I-(aq) + Zn2+(aq)
C) 2I-(aq) + Zn(s) → I2(s) + Zn2+(aq)
D) I2(s) + Zn2+(aq) → 2I-(aq) + Zn(s)
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
7
Which one of the following statements about electrochemical cells is correct?
A) In a salt bridge, current is carried by cations moving toward the anode, and anions toward the cathode.
B) In the external wire, electrons travel from cathode to anode.
C) The anode of a voltaic cell is labeled minus (-).
D) Oxidation occurs at the cathode, in an electrolytic cell.
E) None of these choices are correct.
A) In a salt bridge, current is carried by cations moving toward the anode, and anions toward the cathode.
B) In the external wire, electrons travel from cathode to anode.
C) The anode of a voltaic cell is labeled minus (-).
D) Oxidation occurs at the cathode, in an electrolytic cell.
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
8
Consider the following balanced redox reaction Mn2+(aq) + S2O82-(aq) + 2H2O(l) → MnO2(s) + 4H(aq) + 2SO42-(aq)
Which of the following statements is true?
A) Mn2+(aq) is the oxidizing agent and is reduced.
B) Mn2+(aq) is the oxidizing agent and is oxidized.
C) Mn2+(aq) is the reducing agent and is oxidized.
D) Mn2+(aq) is the reducing agent and is reduced.
E) Manganese does not change its oxidation number in this reaction.
Which of the following statements is true?
A) Mn2+(aq) is the oxidizing agent and is reduced.
B) Mn2+(aq) is the oxidizing agent and is oxidized.
C) Mn2+(aq) is the reducing agent and is oxidized.
D) Mn2+(aq) is the reducing agent and is reduced.
E) Manganese does not change its oxidation number in this reaction.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
9
When the following redox equation is balanced with smallest whole number coefficients, the coefficient for Sn(OH)3-will be Bi(OH)3(s) + Sn(OH)3(aq) → Sn(OH)62-(aq) + Bi(s) (basic solution)
A) 1.
B) 2.
C) 3.
D) 6.
E) None of these choices are correct.
A) 1.
B) 2.
C) 3.
D) 6.
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
10
Consider the reaction CuO(s) + H2(g) → Cu(s) + H2O(l)
In this reaction, which substances are the oxidant and reductant, respectively?
A) CuO and H2
B) H2 and CuO
C) CuO and Cu
D) H2O and H2
E) None of these choices are correct.
In this reaction, which substances are the oxidant and reductant, respectively?
A) CuO and H2
B) H2 and CuO
C) CuO and Cu
D) H2O and H2
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
11
Which component of the following cell notation is the anode? P |  || R | S
|| R | S
A) P
B)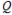
C) R
D) S
E) One of the | symbols is the anode.
 || R | S
|| R | SA) P
B)
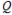
C) R
D) S
E) One of the | symbols is the anode.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
12
When the following redox equation is balanced with smallest whole number coefficients, the coefficient for nitrogen dioxide will be--. I2(s) + HNO3(aq) → HIO3(aq) + NO2(g) + H2O(l)
A) 1
B) 2
C) 4
D) 10
E) None of these choices are correct.
A) 1
B) 2
C) 4
D) 10
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
13
When the following redox equation is balanced with smallest whole number coefficients, the coefficient for the iodide ion will be I-(aq) + NO3-(aq) → NO(g) + I2(s) (acidic solution)
A) 2.
B) 3.
C) 6.
D) 8.
E) None of these choices are correct.
A) 2.
B) 3.
C) 6.
D) 8.
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
14
Which one of the following pairs of substances could be used to construct a single redox electrode (i.e., they have an element in common, but in different oxidation states)?
A) HCl and Cl-
B) H+ and OH-
C) H2O and H+
D) Fe3+ and Fe2O3
E) MnO2 and Mn2+
A) HCl and Cl-
B) H+ and OH-
C) H2O and H+
D) Fe3+ and Fe2O3
E) MnO2 and Mn2+

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following statements about voltaic and electrolytic cells is correct?
A) The electrons in the external wire flow from cathode to anode in both types of cell.
B) Oxidation occurs at the cathode only in a voltaic cell.
C) The free energy change, ΔG, is negative for an electrolytic cell.
D) The cathode is labeled as positive (+) in a voltaic cell but negative (-) in an electrolytic cell.
E) Reduction occurs at the anode in an electrolytic cell.
A) The electrons in the external wire flow from cathode to anode in both types of cell.
B) Oxidation occurs at the cathode only in a voltaic cell.
C) The free energy change, ΔG, is negative for an electrolytic cell.
D) The cathode is labeled as positive (+) in a voltaic cell but negative (-) in an electrolytic cell.
E) Reduction occurs at the anode in an electrolytic cell.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
16
Consider the following redox equation Mn(OH)2(s) + MnO4(aq) → MnO42-(aq) (basic solution)
When the equation is balanced with smallest whole number coefficients, what is the coefficient for OH-(aq) and on which side of the equation is OH-(aq) present?
A) 4, reactant side
B) 4, product side
C) 6, reactant side
D) 6, product side
E) None of these choices are correct.
When the equation is balanced with smallest whole number coefficients, what is the coefficient for OH-(aq) and on which side of the equation is OH-(aq) present?
A) 4, reactant side
B) 4, product side
C) 6, reactant side
D) 6, product side
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
17
A voltaic cell prepared using aluminum and nickel has the following cell notation. Al(s) | Al3+(aq) || Ni2+(aq) | Ni(s)
Which of the following represents the correctly balanced spontaneous reaction equation for the cell?
A) Ni2+(aq) + Al(s) → Al3+(aq) + Ni(s)
B) 3Ni2+(aq) + 2Al(s) → 2Al3+(aq) + 3Ni(s)
C) Ni(s) + Al3+(aq) → Ni2+(aq) + Al(s)
D) 3Ni(s) + 2Al3+(aq) → 3Ni2+(aq) + 2Al(s)
E) None of these choices are correct.
Which of the following represents the correctly balanced spontaneous reaction equation for the cell?
A) Ni2+(aq) + Al(s) → Al3+(aq) + Ni(s)
B) 3Ni2+(aq) + 2Al(s) → 2Al3+(aq) + 3Ni(s)
C) Ni(s) + Al3+(aq) → Ni2+(aq) + Al(s)
D) 3Ni(s) + 2Al3+(aq) → 3Ni2+(aq) + 2Al(s)
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
18
Consider the following balanced redox reaction 3CuO(s) + 2NH3(aq) → N2(g) + 3H2O(l) + 3Cu(s)
Which of the following statements is true?
A) CuO(s) is the oxidizing agent and copper is reduced.
B) CuO(s) is the oxidizing agent and copper is oxidized.
C) CuO(s) is the reducing agent and copper is oxidized.
D) CuO(s) is the reducing agent and copper is reduced.
E) CuO(s) is the oxidizing agent and N2(g) is the reducing agent.
Which of the following statements is true?
A) CuO(s) is the oxidizing agent and copper is reduced.
B) CuO(s) is the oxidizing agent and copper is oxidized.
C) CuO(s) is the reducing agent and copper is oxidized.
D) CuO(s) is the reducing agent and copper is reduced.
E) CuO(s) is the oxidizing agent and N2(g) is the reducing agent.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
19
Which one of the following is not a redox reaction?
A) Al(OH)4(aq) + 4H(aq) → Al3+(aq) + 4H2O(l)
B) C6H12O6(s) + 6O2(g) → 6CO2(g) + 6H2O(l)
C) Na6FeCl8(s) + 2Na(l) → 8NaCl(s) + Fe(s)
D) 2H2O2(aq) → 2H2O(l) + O2(g)
E) CO2(g) + H2(g) → CO(g) + H2O(g)
A) Al(OH)4(aq) + 4H(aq) → Al3+(aq) + 4H2O(l)
B) C6H12O6(s) + 6O2(g) → 6CO2(g) + 6H2O(l)
C) Na6FeCl8(s) + 2Na(l) → 8NaCl(s) + Fe(s)
D) 2H2O2(aq) → 2H2O(l) + O2(g)
E) CO2(g) + H2(g) → CO(g) + H2O(g)

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following solids is commonly used as an inactive electrode in electrochemical cells?
A) zinc
B) graphite
C) copper
D) iron
E) sodium
A) zinc
B) graphite
C) copper
D) iron
E) sodium

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
21
The redox reaction of peroxydisulfate with iodide has been used for many years as part of the iodine clock reaction which introduces students to kinetics. If E°cell = 1.587 V and E° of the cathode half-cell is 0.536 V, what is E° of the anode half-cell? S2O82-(aq) + 2H+ + 2I-(aq) → 2HSO4-(aq) + I2(aq)
A) -1.051 V
B) -2.123 V
C) 1.051 V
D) 2.123 V
E) None of these choices are correct.
A) -1.051 V
B) -2.123 V
C) 1.051 V
D) 2.123 V
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
22
Examine the following half-reactions and select the strongest oxidizing agent among the species listed. Cr2+(aq) + 2e-
 Cr(s) E° = -0.913 V
Cr(s) E° = -0.913 V
Fe2+(aq) + 2e-
 Fe(s) E° = -0.447 V
Fe(s) E° = -0.447 V
Sr2+(aq) + 2e-
 Sr(s) E° = -2.89 V
Sr(s) E° = -2.89 V
Co2+(aq) + 2e-
 Co(s) E° = -0.28 V
Co(s) E° = -0.28 V
A) Cr2+(aq)
B) Fe(s)
C) Fe2+(aq)
D) Sr2+(aq)
E) Co2+(aq)
 Cr(s) E° = -0.913 V
Cr(s) E° = -0.913 VFe2+(aq) + 2e-
 Fe(s) E° = -0.447 V
Fe(s) E° = -0.447 VSr2+(aq) + 2e-
 Sr(s) E° = -2.89 V
Sr(s) E° = -2.89 VCo2+(aq) + 2e-
 Co(s) E° = -0.28 V
Co(s) E° = -0.28 VA) Cr2+(aq)
B) Fe(s)
C) Fe2+(aq)
D) Sr2+(aq)
E) Co2+(aq)

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
23
A voltaic cell has a standard cell potential equal to 0.74 V. If the standard electrode (reduction) potential for the anode is -0.22 V, what is the standard electrode potential for the cathode?
A) 0.96 V
B) 0.52 V
C) -0.52 V
D) -0.96 V
E) Need to know the cell reaction in order to calculate the answer.
A) 0.96 V
B) 0.52 V
C) -0.52 V
D) -0.96 V
E) Need to know the cell reaction in order to calculate the answer.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
24
The voltaic cell made up of cobalt, copper, and their M2+ ions, has E°cell = 0.62 V. If E° of the cathode half-cell is 0.34 V, what is E° of the anode half-cell? Cu2+(aq) + Co(s) → Cu(s) + Co2+(aq)
A) -0.28 V
B) -0.96 V
C) 0.28 V
D) 0.96 V
E) None of these choices are correct.
A) -0.28 V
B) -0.96 V
C) 0.28 V
D) 0.96 V
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following conditions is most likely to apply to a fully-charged secondary cell?
A) E cell l = E° cell
B) E° cell = 0
C)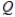 = 1
= 1
D)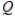 < K
< K
E)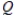 = K
= K
A) E cell l = E° cell
B) E° cell = 0
C)
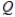 = 1
= 1D)
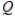 < K
< KE)
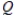 = K
= K
Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
26
When metal A is placed in a solution of metal ions B2+, a reaction occurs between A and B2+, and metal ions A2+ appear in the solution. When metal B is placed in acid solution, gas bubbles form on its surface. When metal A is placed in a solution of metal ions C2+, no reaction occurs. Which of the following reactions would not occur spontaneously?
A) C(s) + 2H+(aq) → H2(g) + C+(aq)
B) C(s) + A2+(aq) → A(s) + C2+(aq)
C) B(s) + C2+(aq) → C(s) + B2+(aq)
D) A(s) + 2H+(aq) → H2(g) + A2+(aq)
E) B(s) + 2H+(aq) → H2(g) + B2+(aq)
A) C(s) + 2H+(aq) → H2(g) + C+(aq)
B) C(s) + A2+(aq) → A(s) + C2+(aq)
C) B(s) + C2+(aq) → C(s) + B2+(aq)
D) A(s) + 2H+(aq) → H2(g) + A2+(aq)
E) B(s) + 2H+(aq) → H2(g) + B2+(aq)

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
27
Examine the following half-reactions and select the strongest reducing agent among the species listed. HgO(s) + H2O(l) + 2e-
 Hg(l) + 2OH-(aq) E°= 0.0977 V
Hg(l) + 2OH-(aq) E°= 0.0977 V
Zn(OH)2(s) + 2e-
 Zn(s) + 2OH-(aq) E° = -1.25 V
Zn(s) + 2OH-(aq) E° = -1.25 V
Ag2O(s) + H2O(l) + 2e-
 Ag(s) + 2OH-(aq) E° = 0.342 V
Ag(s) + 2OH-(aq) E° = 0.342 V
B(OH)3(aq) + 7H+(aq) + 8e-
 BH4-(aq) + 3H2O(l) E° = -0.481 V
BH4-(aq) + 3H2O(l) E° = -0.481 V
A) Hg(l)
B) Zn(s)
C) Ag(s)
D) BH4-(aq)
E) Zn(OH)2(s)
 Hg(l) + 2OH-(aq) E°= 0.0977 V
Hg(l) + 2OH-(aq) E°= 0.0977 VZn(OH)2(s) + 2e-
 Zn(s) + 2OH-(aq) E° = -1.25 V
Zn(s) + 2OH-(aq) E° = -1.25 VAg2O(s) + H2O(l) + 2e-
 Ag(s) + 2OH-(aq) E° = 0.342 V
Ag(s) + 2OH-(aq) E° = 0.342 VB(OH)3(aq) + 7H+(aq) + 8e-
 BH4-(aq) + 3H2O(l) E° = -0.481 V
BH4-(aq) + 3H2O(l) E° = -0.481 VA) Hg(l)
B) Zn(s)
C) Ag(s)
D) BH4-(aq)
E) Zn(OH)2(s)

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
28
Calculate E°cell and indicate whether the overall reaction shown is spontaneous or nonspontaneous. Co3+(aq) + e-
 Co2+(aq) E° = 1.82 V
Co2+(aq) E° = 1.82 V
MnO4-(aq) + 2H2O(l) + 3e- MnO2(s) + 4OH-(aq) E° = 0.59 V
Overall reaction:
MnO4-(aq) + 2H2O(l) + 3Co2+(aq) → MnO2(s) + 3Co3+(aq) + 4OH-(aq)
A) E° cell = -1.23 V, spontaneous
B) E° cell = -1.23 V, nonspontaneous
C) E° cell = 1.23 V, spontaneous
D) E° cell = 1.23 V, nonspontaneous
E) E° cell = -0.05 V, nonspontaneous
 Co2+(aq) E° = 1.82 V
Co2+(aq) E° = 1.82 VMnO4-(aq) + 2H2O(l) + 3e- MnO2(s) + 4OH-(aq) E° = 0.59 V
Overall reaction:
MnO4-(aq) + 2H2O(l) + 3Co2+(aq) → MnO2(s) + 3Co3+(aq) + 4OH-(aq)
A) E° cell = -1.23 V, spontaneous
B) E° cell = -1.23 V, nonspontaneous
C) E° cell = 1.23 V, spontaneous
D) E° cell = 1.23 V, nonspontaneous
E) E° cell = -0.05 V, nonspontaneous

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
29
What is the E°cell for the cell represented by the combination of the following half-reactions? ClO4(aq) + 8H(aq) + 8e  Cl-(aq) + 4H2O(l) E° = 1.389 V
Cl-(aq) + 4H2O(l) E° = 1.389 V
VO2(aq) + 2H(aq) + e- j VO(aq) + H2O(l) E° = 0.991 V
VO(aq) + H2O(l) E° = 0.991 V
A) -0.398 V
B) -2.380 V
C) 0.398 V
D) 2.380 V
E) None of these choices are correct.
 Cl-(aq) + 4H2O(l) E° = 1.389 V
Cl-(aq) + 4H2O(l) E° = 1.389 VVO2(aq) + 2H(aq) + e- j
 VO(aq) + H2O(l) E° = 0.991 V
VO(aq) + H2O(l) E° = 0.991 VA) -0.398 V
B) -2.380 V
C) 0.398 V
D) 2.380 V
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
30
A voltaic cell can be prepared from copper and tin. What is the E°cell for the cell that forms from the following half-reactions? Cu2+(aq) + 2e-  Cu(s) E° = 0.34 V
Cu(s) E° = 0.34 V
Sn4+(aq) + 2e- Sn2+(aq) E° = 0.13 V
Sn2+(aq) E° = 0.13 V
A) 0.47 V
B) 0.21 V
C) -0.21 V
D) -0.47 V
E) 0.42 V
 Cu(s) E° = 0.34 V
Cu(s) E° = 0.34 VSn4+(aq) + 2e-
 Sn2+(aq) E° = 0.13 V
Sn2+(aq) E° = 0.13 VA) 0.47 V
B) 0.21 V
C) -0.21 V
D) -0.47 V
E) 0.42 V

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
31
Calculate E° cell and indicate whether the overall reaction shown is spontaneous or nonspontaneous. H2O2(aq) + 2H+(aq) + 2e- 2H2O(l) E° = 1.77 V
Fe3+ (aq) + e- Fe2+(aq) E° = 0.77 V
Overall reaction:
2Fe3+(aq) + 2H2O(l) → H2O2(aq) + 2H+(aq) + 2Fe2+(aq)
A) E° cell = -1.00 V, nonspontaneous
B) E° cell = -1.00 V, spontaneous
C) E° cell = 1.00 V, nonspontaneous
D) E° cell = 1.00 V, spontaneous
E) E° cell = -0.23 V, nonspontaneous
Fe3+ (aq) + e- Fe2+(aq) E° = 0.77 V
Overall reaction:
2Fe3+(aq) + 2H2O(l) → H2O2(aq) + 2H+(aq) + 2Fe2+(aq)
A) E° cell = -1.00 V, nonspontaneous
B) E° cell = -1.00 V, spontaneous
C) E° cell = 1.00 V, nonspontaneous
D) E° cell = 1.00 V, spontaneous
E) E° cell = -0.23 V, nonspontaneous

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
32
The line notation, Al(s) | Al3+(aq) || Co2+(aq) | Co(s), indicates that
A) Co is the reducing agent.
B) Co2+ ions are oxidized.
C) Al is the reducing agent.
D) Al3+ is the reducing agent.
E) aluminum metal is the cathode.
A) Co is the reducing agent.
B) Co2+ ions are oxidized.
C) Al is the reducing agent.
D) Al3+ is the reducing agent.
E) aluminum metal is the cathode.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
33
Examine the following half-reactions and select the strongest oxidizing agent among the substances. [PtCl4]2-(aq) + 2e-
![<strong>Examine the following half-reactions and select the strongest oxidizing agent among the substances. [PtCl<sub>4</sub>]<sup>2-</sup>(aq) + 2e<sup>-</sup> <sup> </sup> Pt(s) + 4Cl<sup>-</sup>(aq) E° = 0.755 V RuO<sub>4</sub>(s) + 8H<sup>+</sup>(aq) + 8e<sup>-</sup> <sup> </sup> Ru(s) + 4H<sub>2</sub>O(l) E° = 1.038 V FeO<sub>4</sub><sup>2-</sup>(aq) + 8H<sup>+</sup>(aq) + 3e<sup>-</sup> <sup> </sup> Fe<sup>3+</sup>(aq) + 4H<sub>2</sub>O(l) E° = 2.07 V H<sub>4</sub>XeO<sub>6</sub>(aq) + 2H<sup>+</sup>(aq) + 2e<sup>-</sup> <sup> </sup> XeO<sub>3</sub>(aq) + 3H<sub>2</sub>O(l) E° = 2.42 V</strong> A) [PtCl<sub>4</sub>]<sup>2-</sup>(aq) B) RuO<sub>4</sub>(s) C) HFeO<sub>4</sub><sup>-</sup> (aq) D) H<sub>4</sub>XeO<sub>6</sub>(aq) E) Cl<sup>-</sup>(aq)](https://d2lvgg3v3hfg70.cloudfront.net/TB7800/11eac68a_1c38_7599_a448_297c6d6618ad_TB7800_11.jpg) Pt(s) + 4Cl-(aq) E° = 0.755 V
Pt(s) + 4Cl-(aq) E° = 0.755 V
RuO4(s) + 8H+(aq) + 8e-
![<strong>Examine the following half-reactions and select the strongest oxidizing agent among the substances. [PtCl<sub>4</sub>]<sup>2-</sup>(aq) + 2e<sup>-</sup> <sup> </sup> Pt(s) + 4Cl<sup>-</sup>(aq) E° = 0.755 V RuO<sub>4</sub>(s) + 8H<sup>+</sup>(aq) + 8e<sup>-</sup> <sup> </sup> Ru(s) + 4H<sub>2</sub>O(l) E° = 1.038 V FeO<sub>4</sub><sup>2-</sup>(aq) + 8H<sup>+</sup>(aq) + 3e<sup>-</sup> <sup> </sup> Fe<sup>3+</sup>(aq) + 4H<sub>2</sub>O(l) E° = 2.07 V H<sub>4</sub>XeO<sub>6</sub>(aq) + 2H<sup>+</sup>(aq) + 2e<sup>-</sup> <sup> </sup> XeO<sub>3</sub>(aq) + 3H<sub>2</sub>O(l) E° = 2.42 V</strong> A) [PtCl<sub>4</sub>]<sup>2-</sup>(aq) B) RuO<sub>4</sub>(s) C) HFeO<sub>4</sub><sup>-</sup> (aq) D) H<sub>4</sub>XeO<sub>6</sub>(aq) E) Cl<sup>-</sup>(aq)](https://d2lvgg3v3hfg70.cloudfront.net/TB7800/11eac68a_1c38_759a_a448_91b568d809f7_TB7800_11.jpg) Ru(s) + 4H2O(l) E° = 1.038 V
Ru(s) + 4H2O(l) E° = 1.038 V
FeO42-(aq) + 8H+(aq) + 3e-
![<strong>Examine the following half-reactions and select the strongest oxidizing agent among the substances. [PtCl<sub>4</sub>]<sup>2-</sup>(aq) + 2e<sup>-</sup> <sup> </sup> Pt(s) + 4Cl<sup>-</sup>(aq) E° = 0.755 V RuO<sub>4</sub>(s) + 8H<sup>+</sup>(aq) + 8e<sup>-</sup> <sup> </sup> Ru(s) + 4H<sub>2</sub>O(l) E° = 1.038 V FeO<sub>4</sub><sup>2-</sup>(aq) + 8H<sup>+</sup>(aq) + 3e<sup>-</sup> <sup> </sup> Fe<sup>3+</sup>(aq) + 4H<sub>2</sub>O(l) E° = 2.07 V H<sub>4</sub>XeO<sub>6</sub>(aq) + 2H<sup>+</sup>(aq) + 2e<sup>-</sup> <sup> </sup> XeO<sub>3</sub>(aq) + 3H<sub>2</sub>O(l) E° = 2.42 V</strong> A) [PtCl<sub>4</sub>]<sup>2-</sup>(aq) B) RuO<sub>4</sub>(s) C) HFeO<sub>4</sub><sup>-</sup> (aq) D) H<sub>4</sub>XeO<sub>6</sub>(aq) E) Cl<sup>-</sup>(aq)](https://d2lvgg3v3hfg70.cloudfront.net/TB7800/11eac68a_1c38_9cab_a448_0da23547da41_TB7800_11.jpg) Fe3+(aq) + 4H2O(l) E° = 2.07 V
Fe3+(aq) + 4H2O(l) E° = 2.07 V
H4XeO6(aq) + 2H+(aq) + 2e-
![<strong>Examine the following half-reactions and select the strongest oxidizing agent among the substances. [PtCl<sub>4</sub>]<sup>2-</sup>(aq) + 2e<sup>-</sup> <sup> </sup> Pt(s) + 4Cl<sup>-</sup>(aq) E° = 0.755 V RuO<sub>4</sub>(s) + 8H<sup>+</sup>(aq) + 8e<sup>-</sup> <sup> </sup> Ru(s) + 4H<sub>2</sub>O(l) E° = 1.038 V FeO<sub>4</sub><sup>2-</sup>(aq) + 8H<sup>+</sup>(aq) + 3e<sup>-</sup> <sup> </sup> Fe<sup>3+</sup>(aq) + 4H<sub>2</sub>O(l) E° = 2.07 V H<sub>4</sub>XeO<sub>6</sub>(aq) + 2H<sup>+</sup>(aq) + 2e<sup>-</sup> <sup> </sup> XeO<sub>3</sub>(aq) + 3H<sub>2</sub>O(l) E° = 2.42 V</strong> A) [PtCl<sub>4</sub>]<sup>2-</sup>(aq) B) RuO<sub>4</sub>(s) C) HFeO<sub>4</sub><sup>-</sup> (aq) D) H<sub>4</sub>XeO<sub>6</sub>(aq) E) Cl<sup>-</sup>(aq)](https://d2lvgg3v3hfg70.cloudfront.net/TB7800/11eac68a_1c38_9cac_a448_6fe5003b71f4_TB7800_11.jpg) XeO3(aq) + 3H2O(l) E° = 2.42 V
XeO3(aq) + 3H2O(l) E° = 2.42 V
A) [PtCl4]2-(aq)
B) RuO4(s)
C) HFeO4- (aq)
D) H4XeO6(aq)
E) Cl-(aq)
![<strong>Examine the following half-reactions and select the strongest oxidizing agent among the substances. [PtCl<sub>4</sub>]<sup>2-</sup>(aq) + 2e<sup>-</sup> <sup> </sup> Pt(s) + 4Cl<sup>-</sup>(aq) E° = 0.755 V RuO<sub>4</sub>(s) + 8H<sup>+</sup>(aq) + 8e<sup>-</sup> <sup> </sup> Ru(s) + 4H<sub>2</sub>O(l) E° = 1.038 V FeO<sub>4</sub><sup>2-</sup>(aq) + 8H<sup>+</sup>(aq) + 3e<sup>-</sup> <sup> </sup> Fe<sup>3+</sup>(aq) + 4H<sub>2</sub>O(l) E° = 2.07 V H<sub>4</sub>XeO<sub>6</sub>(aq) + 2H<sup>+</sup>(aq) + 2e<sup>-</sup> <sup> </sup> XeO<sub>3</sub>(aq) + 3H<sub>2</sub>O(l) E° = 2.42 V</strong> A) [PtCl<sub>4</sub>]<sup>2-</sup>(aq) B) RuO<sub>4</sub>(s) C) HFeO<sub>4</sub><sup>-</sup> (aq) D) H<sub>4</sub>XeO<sub>6</sub>(aq) E) Cl<sup>-</sup>(aq)](https://d2lvgg3v3hfg70.cloudfront.net/TB7800/11eac68a_1c38_7599_a448_297c6d6618ad_TB7800_11.jpg) Pt(s) + 4Cl-(aq) E° = 0.755 V
Pt(s) + 4Cl-(aq) E° = 0.755 VRuO4(s) + 8H+(aq) + 8e-
![<strong>Examine the following half-reactions and select the strongest oxidizing agent among the substances. [PtCl<sub>4</sub>]<sup>2-</sup>(aq) + 2e<sup>-</sup> <sup> </sup> Pt(s) + 4Cl<sup>-</sup>(aq) E° = 0.755 V RuO<sub>4</sub>(s) + 8H<sup>+</sup>(aq) + 8e<sup>-</sup> <sup> </sup> Ru(s) + 4H<sub>2</sub>O(l) E° = 1.038 V FeO<sub>4</sub><sup>2-</sup>(aq) + 8H<sup>+</sup>(aq) + 3e<sup>-</sup> <sup> </sup> Fe<sup>3+</sup>(aq) + 4H<sub>2</sub>O(l) E° = 2.07 V H<sub>4</sub>XeO<sub>6</sub>(aq) + 2H<sup>+</sup>(aq) + 2e<sup>-</sup> <sup> </sup> XeO<sub>3</sub>(aq) + 3H<sub>2</sub>O(l) E° = 2.42 V</strong> A) [PtCl<sub>4</sub>]<sup>2-</sup>(aq) B) RuO<sub>4</sub>(s) C) HFeO<sub>4</sub><sup>-</sup> (aq) D) H<sub>4</sub>XeO<sub>6</sub>(aq) E) Cl<sup>-</sup>(aq)](https://d2lvgg3v3hfg70.cloudfront.net/TB7800/11eac68a_1c38_759a_a448_91b568d809f7_TB7800_11.jpg) Ru(s) + 4H2O(l) E° = 1.038 V
Ru(s) + 4H2O(l) E° = 1.038 VFeO42-(aq) + 8H+(aq) + 3e-
![<strong>Examine the following half-reactions and select the strongest oxidizing agent among the substances. [PtCl<sub>4</sub>]<sup>2-</sup>(aq) + 2e<sup>-</sup> <sup> </sup> Pt(s) + 4Cl<sup>-</sup>(aq) E° = 0.755 V RuO<sub>4</sub>(s) + 8H<sup>+</sup>(aq) + 8e<sup>-</sup> <sup> </sup> Ru(s) + 4H<sub>2</sub>O(l) E° = 1.038 V FeO<sub>4</sub><sup>2-</sup>(aq) + 8H<sup>+</sup>(aq) + 3e<sup>-</sup> <sup> </sup> Fe<sup>3+</sup>(aq) + 4H<sub>2</sub>O(l) E° = 2.07 V H<sub>4</sub>XeO<sub>6</sub>(aq) + 2H<sup>+</sup>(aq) + 2e<sup>-</sup> <sup> </sup> XeO<sub>3</sub>(aq) + 3H<sub>2</sub>O(l) E° = 2.42 V</strong> A) [PtCl<sub>4</sub>]<sup>2-</sup>(aq) B) RuO<sub>4</sub>(s) C) HFeO<sub>4</sub><sup>-</sup> (aq) D) H<sub>4</sub>XeO<sub>6</sub>(aq) E) Cl<sup>-</sup>(aq)](https://d2lvgg3v3hfg70.cloudfront.net/TB7800/11eac68a_1c38_9cab_a448_0da23547da41_TB7800_11.jpg) Fe3+(aq) + 4H2O(l) E° = 2.07 V
Fe3+(aq) + 4H2O(l) E° = 2.07 VH4XeO6(aq) + 2H+(aq) + 2e-
![<strong>Examine the following half-reactions and select the strongest oxidizing agent among the substances. [PtCl<sub>4</sub>]<sup>2-</sup>(aq) + 2e<sup>-</sup> <sup> </sup> Pt(s) + 4Cl<sup>-</sup>(aq) E° = 0.755 V RuO<sub>4</sub>(s) + 8H<sup>+</sup>(aq) + 8e<sup>-</sup> <sup> </sup> Ru(s) + 4H<sub>2</sub>O(l) E° = 1.038 V FeO<sub>4</sub><sup>2-</sup>(aq) + 8H<sup>+</sup>(aq) + 3e<sup>-</sup> <sup> </sup> Fe<sup>3+</sup>(aq) + 4H<sub>2</sub>O(l) E° = 2.07 V H<sub>4</sub>XeO<sub>6</sub>(aq) + 2H<sup>+</sup>(aq) + 2e<sup>-</sup> <sup> </sup> XeO<sub>3</sub>(aq) + 3H<sub>2</sub>O(l) E° = 2.42 V</strong> A) [PtCl<sub>4</sub>]<sup>2-</sup>(aq) B) RuO<sub>4</sub>(s) C) HFeO<sub>4</sub><sup>-</sup> (aq) D) H<sub>4</sub>XeO<sub>6</sub>(aq) E) Cl<sup>-</sup>(aq)](https://d2lvgg3v3hfg70.cloudfront.net/TB7800/11eac68a_1c38_9cac_a448_6fe5003b71f4_TB7800_11.jpg) XeO3(aq) + 3H2O(l) E° = 2.42 V
XeO3(aq) + 3H2O(l) E° = 2.42 VA) [PtCl4]2-(aq)
B) RuO4(s)
C) HFeO4- (aq)
D) H4XeO6(aq)
E) Cl-(aq)

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
34
Calculate E°cell and indicate whether the overall reaction shown is spontaneous or nonspontaneous. I2(s) + 2e-
 2I-(aq) E° = 0.53 V
2I-(aq) E° = 0.53 V
Cr3+(aq) + 3e-
 Cr(s) E° = -0.74 V
Cr(s) E° = -0.74 V
Overall reaction:
2Cr(s) + 3I2(s) → 2Cr3+(aq) + (aq) + 6I-(aq)
A) E°cell = -1.27 V, spontaneous
B) E° cell = -1.27 V, nonspontaneous
C) E° cell = 1.27 V, spontaneous
D) E° cell = 1.27 V, nonspontaneous
E) E° cell = 1.54 V, spontaneous
 2I-(aq) E° = 0.53 V
2I-(aq) E° = 0.53 VCr3+(aq) + 3e-
 Cr(s) E° = -0.74 V
Cr(s) E° = -0.74 VOverall reaction:
2Cr(s) + 3I2(s) → 2Cr3+(aq) + (aq) + 6I-(aq)
A) E°cell = -1.27 V, spontaneous
B) E° cell = -1.27 V, nonspontaneous
C) E° cell = 1.27 V, spontaneous
D) E° cell = 1.27 V, nonspontaneous
E) E° cell = 1.54 V, spontaneous

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
35
Calculate E° cell and indicate whether the overall reaction shown is spontaneous or nonspontaneous. O2(g) + 4H+(aq) + 4e-
 2H2O(l) E° = 1.229 V
2H2O(l) E° = 1.229 V
Al3+(aq) + 3e-
 Al(s) E° = -1.662 V
Al(s) E° = -1.662 V
Overall reaction:
4Al(s) + 3O2(g) + 12H+(aq) → 4Al3+(aq) + 6H2O(l)
A) E° cell = -2.891 V, nonspontaneous
B) E° cell = -2.891 V, spontaneous
C) E° cell = 2.891 V, nonspontaneous
D) E° cell = 2.891 V, spontaneous
E) None of these choices are correct.
 2H2O(l) E° = 1.229 V
2H2O(l) E° = 1.229 VAl3+(aq) + 3e-
 Al(s) E° = -1.662 V
Al(s) E° = -1.662 VOverall reaction:
4Al(s) + 3O2(g) + 12H+(aq) → 4Al3+(aq) + 6H2O(l)
A) E° cell = -2.891 V, nonspontaneous
B) E° cell = -2.891 V, spontaneous
C) E° cell = 2.891 V, nonspontaneous
D) E° cell = 2.891 V, spontaneous
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
36
Examine the following half-reactions and select the weakest oxidizing agent among the species listed. AuBr4(aq) + 3e-
 Au(s) + 4Br-(aq) E° = 0.854 V
Au(s) + 4Br-(aq) E° = 0.854 V
Mn2+(aq) + 2e-
 Mn(s) E° = -1.185 V
Mn(s) E° = -1.185 V
K+(aq) + e-
 K(s) E° = -2.931 V
K(s) E° = -2.931 V
F2O(aq) + 2H+(aq) + 4e-
 2F-(aq) + H2O(l) E° = 2.153 V
2F-(aq) + H2O(l) E° = 2.153 V
A) AuBr4-(aq)
B) Mn2+(aq)
C) K+(aq)
D) F2O(aq)
E) H+(aq)
 Au(s) + 4Br-(aq) E° = 0.854 V
Au(s) + 4Br-(aq) E° = 0.854 VMn2+(aq) + 2e-
 Mn(s) E° = -1.185 V
Mn(s) E° = -1.185 VK+(aq) + e-
 K(s) E° = -2.931 V
K(s) E° = -2.931 VF2O(aq) + 2H+(aq) + 4e-
 2F-(aq) + H2O(l) E° = 2.153 V
2F-(aq) + H2O(l) E° = 2.153 VA) AuBr4-(aq)
B) Mn2+(aq)
C) K+(aq)
D) F2O(aq)
E) H+(aq)

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
37
What is the E°cell for the cell represented by the combination of the following half-reactions? 2Hg2+(aq) + 2e-  Hg22+(aq) E° = 0.92 V
Hg22+(aq) E° = 0.92 V
Cr3+(aq) + 3e- Cr(s) E° = -0.74 V
Cr(s) E° = -0.74 V
A) -0.18 V
B) 0.18 V
C) 1.28 V
D) 1.66 V
E) 2.12 V
 Hg22+(aq) E° = 0.92 V
Hg22+(aq) E° = 0.92 VCr3+(aq) + 3e-
 Cr(s) E° = -0.74 V
Cr(s) E° = -0.74 VA) -0.18 V
B) 0.18 V
C) 1.28 V
D) 1.66 V
E) 2.12 V

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
38
Examine the following half-reactions and select the strongest reducing agent among the species listed. PbI2(s) + 2e-
 Pb(s) + 2I-(aq) E° = -0.365 V
Pb(s) + 2I-(aq) E° = -0.365 V
Ca2+(aq) + 2e-
 Ca(s) E° = -2.868 V
Ca(s) E° = -2.868 V
Pt2+(aq) + 2e-
 Pt(s) E° = 1.18 V
Pt(s) E° = 1.18 V
Br2(l) + 2e-
 2Br-(aq) E° = 1.066 V
2Br-(aq) E° = 1.066 V
A) Pb(s)
B) Ca(s)
C) Pt(s)
D) Br-(aq)
E) Pt2+(aq)
 Pb(s) + 2I-(aq) E° = -0.365 V
Pb(s) + 2I-(aq) E° = -0.365 VCa2+(aq) + 2e-
 Ca(s) E° = -2.868 V
Ca(s) E° = -2.868 VPt2+(aq) + 2e-
 Pt(s) E° = 1.18 V
Pt(s) E° = 1.18 VBr2(l) + 2e-
 2Br-(aq) E° = 1.066 V
2Br-(aq) E° = 1.066 VA) Pb(s)
B) Ca(s)
C) Pt(s)
D) Br-(aq)
E) Pt2+(aq)

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
39
The line notation, Pt | H2(g) | H+(aq) || Cu2+(aq) | Cu(s), indicates that
A) copper metal is a product of the cell reaction.
B) hydrogen gas (H2) is a product of the cell reaction.
C) Cu is the anode.
D) Pt is the cathode.
E) Cu2+ is the reducing agent.
A) copper metal is a product of the cell reaction.
B) hydrogen gas (H2) is a product of the cell reaction.
C) Cu is the anode.
D) Pt is the cathode.
E) Cu2+ is the reducing agent.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
40
Examine the following half-reactions and select the weakest reducing agent among the substances. Cr(OH)3(s) + 3e-
 Cr(s) + 3OH-(aq) E° = -1.48 V
Cr(s) + 3OH-(aq) E° = -1.48 V
SnO2(s) + 2H2O(l) + 4e-
 Sn(s) + 4OH-(aq) E° = -0.945 V
Sn(s) + 4OH-(aq) E° = -0.945 V
MnO2(s) + 4H+(aq) + 2e-
 Mn2+(aq) + 2H2O(l) E° = 1.224 V
Mn2+(aq) + 2H2O(l) E° = 1.224 V
Hg2SO4(s) + 2e-
 2Hg(l) + SO42-(aq) E° = 0.613 V
2Hg(l) + SO42-(aq) E° = 0.613 V
A) Cr(s)
B) Sn(s)
C) Mn2+(aq)
D) Hg(l)
E) OH-(aq)
 Cr(s) + 3OH-(aq) E° = -1.48 V
Cr(s) + 3OH-(aq) E° = -1.48 VSnO2(s) + 2H2O(l) + 4e-
 Sn(s) + 4OH-(aq) E° = -0.945 V
Sn(s) + 4OH-(aq) E° = -0.945 VMnO2(s) + 4H+(aq) + 2e-
 Mn2+(aq) + 2H2O(l) E° = 1.224 V
Mn2+(aq) + 2H2O(l) E° = 1.224 VHg2SO4(s) + 2e-
 2Hg(l) + SO42-(aq) E° = 0.613 V
2Hg(l) + SO42-(aq) E° = 0.613 VA) Cr(s)
B) Sn(s)
C) Mn2+(aq)
D) Hg(l)
E) OH-(aq)

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
41
Consider the reaction in the lead-acid cell Pb(s) + PbO2(s) + 2H2SO4(aq) → 2PbSO4(aq) + 2H2O(l)
For which E° cell = 2.04 V at 298 K. ΔG° for this reaction is
A) -3.94 × 105 kJ.
B) -3.94 × 102 kJ.
C) -1.97 × 105 kJ.
D) -7.87 × 102 kJ.
E) None of these choices are correct.
For which E° cell = 2.04 V at 298 K. ΔG° for this reaction is
A) -3.94 × 105 kJ.
B) -3.94 × 102 kJ.
C) -1.97 × 105 kJ.
D) -7.87 × 102 kJ.
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
42
Consider the nonaqueous cell reaction 2Na(l) + FeCl2(s)  2NaCl(s) + Fe(s)
2NaCl(s) + Fe(s)
For which E° cell = 2.35 V at 200°C. ΔG° at this temperature is
A) 453 kJ.
B) -453 kJ.
C) 907 kJ.
D) -907 kJ.
E) None of these choices are correct.
 2NaCl(s) + Fe(s)
2NaCl(s) + Fe(s)For which E° cell = 2.35 V at 200°C. ΔG° at this temperature is
A) 453 kJ.
B) -453 kJ.
C) 907 kJ.
D) -907 kJ.
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
43
The following half-reactions occur in the mercury battery used in calculators. If E° cell = 1.357 V, calculate the equilibrium constant for the cell reaction at 25°C. (Assume the stoichiometric coefficients in the cell reaction are all equal to 1.) HgO(s) + H2O(l) + 2e-
 Hg(l) + 2OH-(aq)
Hg(l) + 2OH-(aq)
ZnO(s) + H2O(l) + 2e-
 Zn(s) + 2OH-(aq)
Zn(s) + 2OH-(aq)
A) 9.4 × 1022
B) 7.5 × 1045
C) 6.4 × 1063
D) 7.8 × 1091
E) > 9.9 × 1099
 Hg(l) + 2OH-(aq)
Hg(l) + 2OH-(aq)ZnO(s) + H2O(l) + 2e-
 Zn(s) + 2OH-(aq)
Zn(s) + 2OH-(aq)A) 9.4 × 1022
B) 7.5 × 1045
C) 6.4 × 1063
D) 7.8 × 1091
E) > 9.9 × 1099

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
44
A voltaic cell consists of a Mn/Mn2+ electrode (E° = -1.18 V) and a Fe/Fe2+ electrode (E° = -0.44 V). Calculate [Fe2+] if [Mn2+] = 0.050 M and E cell = 0.78 V at 25°C.
A) 0.040 M
B) 0.24 M
C) 1.1 M
D) 1.8 M
E) None of these choices are correct.
A) 0.040 M
B) 0.24 M
C) 1.1 M
D) 1.8 M
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
45
A voltaic cell consists of an Au/Au3+ electrode (E° = 1.50 V) and a Cu/Cu2+ electrode (E° = 0.34 V). Calculate [Au3+] if [Cu2+] = 1.20 M and Ecell = 1.13 V at 25°C.
A) 0.001 M
B) 0.002 M
C) 0.01 M
D) 0.02 M
E) 0.04 M
A) 0.001 M
B) 0.002 M
C) 0.01 M
D) 0.02 M
E) 0.04 M

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
46
A voltaic cell consists of a Hg/Hg22+ electrode (E° = 0.85 V) and a Sn/Sn2+ electrode (E° = -0.14 V). Calculate [Sn2+] if [Hg22+] = 0.24 M and Ecell = 1.04 V at 25°C.
A) 0.0001 M
B) 0.0007 M
C) 0.005 M
D) 0.03 M
E) 0.05 M
A) 0.0001 M
B) 0.0007 M
C) 0.005 M
D) 0.03 M
E) 0.05 M

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
47
A battery is considered "dead" when
A) < 1.
< 1.
B) = 1.
= 1.
C) > 1.
> 1.
D) = K.
= K.
E) /K = 0.
/K = 0.
A)
 < 1.
< 1.B)
 = 1.
= 1.C)
 > 1.
> 1.D)
 = K.
= K.E)
 /K = 0.
/K = 0.
Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
48
A voltaic cell consists of a Cd/Cd2+ electrode (E° = -0.40 V) and a Fe/Fe2+ electrode (E° = -0.44 V). If Ecell = 0 and the temperature is 25°C, what is the ratio [Fe2+]/[Cd2+]?
A) 2 × 101
B) 1 × 101
C) 1
D) 1 × 10-1
E) 5 × 10-2
A) 2 × 101
B) 1 × 101
C) 1
D) 1 × 10-1
E) 5 × 10-2

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
49
A voltaic cell consists of a Ag/Ag+ electrode (E° = 0.80 V) and a Fe2+/Fe3+ electrode (E° = 0.77 V) with the following initial molar concentrations: [Fe2+] = 0.30 M; [Fe3+] = 0.10 M; [Ag+] = 0.30 M. What is the equilibrium concentration of Fe3+? (Assume the anode and cathode solutions are of equal volume, and a temperature of 25°C.)
A) 0.030 M
B) 0.043 M
C) 0.085 M
D) 0.11 M
E) 0.17 M
A) 0.030 M
B) 0.043 M
C) 0.085 M
D) 0.11 M
E) 0.17 M

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
50
Calculate ΔG° for the reaction of iron(II) ions with one mole of permanganate ions. MnO4-(aq) + 8H+(aq) + 5e-  Mn2+(aq) + 4H2O(l) E° = 1.51 V
Mn2+(aq) + 4H2O(l) E° = 1.51 V
Fe3+(aq) + e-
 Fe2+(aq) E°= 0.77 V
Fe2+(aq) E°= 0.77 V
A) -71.4 kJ
B) -286 kJ
C) -357 kJ
D) -428 kJ
E) None of these choices are correct.
 Mn2+(aq) + 4H2O(l) E° = 1.51 V
Mn2+(aq) + 4H2O(l) E° = 1.51 VFe3+(aq) + e-
 Fe2+(aq) E°= 0.77 V
Fe2+(aq) E°= 0.77 VA) -71.4 kJ
B) -286 kJ
C) -357 kJ
D) -428 kJ
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
51
Consider the reaction of iodine with manganese dioxide 3I2(s) + 2MnO2(s) + 8OH-(aq)  6I-(aq) + 2MnO4(aq) + 4H2O(l)
6I-(aq) + 2MnO4(aq) + 4H2O(l)
The equilibrium constant for the overall reaction is 8.30 × 10-7. Calculate E° cell for the reaction at 25°C.
A) -0.36 V
B) -0.18 V
C) -0.12 V
D) -0.060 V
E) None of these choices are correct.
 6I-(aq) + 2MnO4(aq) + 4H2O(l)
6I-(aq) + 2MnO4(aq) + 4H2O(l)The equilibrium constant for the overall reaction is 8.30 × 10-7. Calculate E° cell for the reaction at 25°C.
A) -0.36 V
B) -0.18 V
C) -0.12 V
D) -0.060 V
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
52
The value of E° cell for the reaction 2Cr3+(aq) + 6Hg(l) → 2Cr(s) + 3Hg22+(aq)
Is 1.59 V. Calculate ΔG° for the reaction.
A) -921 kJ
B) -767 kJ
C) -460 kJ
D) -307 kJ
E) None of these choices are correct.
Is 1.59 V. Calculate ΔG° for the reaction.
A) -921 kJ
B) -767 kJ
C) -460 kJ
D) -307 kJ
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
53
Calculate the potential of a voltaic cell (E° cell) if it is required to do 5.43 × 10-3 kJ of work when a charge of 2.50 C is transferred.
A) 2.17 × 103 V
B) 2.17 × 10-3 V
C) 2.17 V
D) 13.6 V
E) 1.36 × 10-2 V
A) 2.17 × 103 V
B) 2.17 × 10-3 V
C) 2.17 V
D) 13.6 V
E) 1.36 × 10-2 V

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
54
What is the value of the equilibrium constant for the cell reaction below at 25°C? E° cell = 0.61 V 2Cr(s) + 3Pb2+(aq)  3Pb(s) + 2Cr3+(aq)
3Pb(s) + 2Cr3+(aq)
A) 4.1 × 1020
B) 8.2 × 1030
C) 3.3 × 1051
D) 7.4 × 1061
E) > 9.9 × 1099
 3Pb(s) + 2Cr3+(aq)
3Pb(s) + 2Cr3+(aq)A) 4.1 × 1020
B) 8.2 × 1030
C) 3.3 × 1051
D) 7.4 × 1061
E) > 9.9 × 1099

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
55
A concentration cell consists of two Zn/Zn2+ electrodes. The electrolyte in compartment A is 0.10 M Zn(NO3)2 and in compartment B is 0.60 M Zn(NO3)2. What is the voltage of the cell at 25°C?
A) 0.010 V
B) 0.020 V
C) 0.023 V
D) 0.046 V
E) None of these choices are correct.
A) 0.010 V
B) 0.020 V
C) 0.023 V
D) 0.046 V
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
56
The value of the equilibrium constant for the reaction of nickel(II) ions with cadmium metal is 1.17 × 105. Calculate ΔG° for the reaction at 25°C.
A) -12.6 kJ
B) -28.9 kJ
C) 12.6 kJ
D) 28.9 kJ
E) None of these choices are correct.
A) -12.6 kJ
B) -28.9 kJ
C) 12.6 kJ
D) 28.9 kJ
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
57
Consider the reaction of iodine with manganese dioxide 3I2(s) + 2MnO2(s) + 8OH-(aq)  6I-(aq) + 2MnO4(aq) + 4H2O(l)
6I-(aq) + 2MnO4(aq) + 4H2O(l)
The equilibrium constant for the overall reaction is 8.30 × 10-7. Calculate ΔG° for the reaction at 25°C.
A) -15.1 kJ
B) -34.7 kJ
C) 15.1 kJ
D) 34.7 kJ
E) None of these choices are correct.
 6I-(aq) + 2MnO4(aq) + 4H2O(l)
6I-(aq) + 2MnO4(aq) + 4H2O(l)The equilibrium constant for the overall reaction is 8.30 × 10-7. Calculate ΔG° for the reaction at 25°C.
A) -15.1 kJ
B) -34.7 kJ
C) 15.1 kJ
D) 34.7 kJ
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
58
What is the value of the equilibrium constant for the cell reaction below at 25°C? E° cell = 0.30 V Sn2+(aq) + Fe(s)  Sn(s) + Fe2+(aq)
Sn(s) + Fe2+(aq)
A) 1.2 × 105
B) 1.4 × 1010
C) 8.6 × 10-6
D) 7.1 × 10-11
E) 2.3 × 1023
 Sn(s) + Fe2+(aq)
Sn(s) + Fe2+(aq)A) 1.2 × 105
B) 1.4 × 1010
C) 8.6 × 10-6
D) 7.1 × 10-11
E) 2.3 × 1023

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
59
Calculate ΔG° for the oxidation of 3 moles of copper by nitric acid. Cu2+(aq) + 2e-
 Cu(s) E° = 0.34 V
Cu(s) E° = 0.34 V
NO3-(aq) + 4H+(aq) + 3e-
 NO(g) + 2H2O(l) E° = 0.957 V
NO(g) + 2H2O(l) E° = 0.957 V
A) -120 kJ
B) -180 kJ
C) -240 kJ
D) -300 kJ
E) -360 kJ
 Cu(s) E° = 0.34 V
Cu(s) E° = 0.34 VNO3-(aq) + 4H+(aq) + 3e-
 NO(g) + 2H2O(l) E° = 0.957 V
NO(g) + 2H2O(l) E° = 0.957 VA) -120 kJ
B) -180 kJ
C) -240 kJ
D) -300 kJ
E) -360 kJ

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
60
Calculate E° cell for the reaction of nickel(II) ions with cadmium metal at 25°C. K = 1.17 × 105 Ni2+(aq) + Cd(s) → Cd2+(aq) + Ni(s)
A) 0.075 V
B) 0.10 V
C) 0.12 V
D) 0.15 V
E) 0.30 V
A) 0.075 V
B) 0.10 V
C) 0.12 V
D) 0.15 V
E) 0.30 V

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
61
Which, if any, of the following metals would be capable of acting as a sacrificial anode when used with iron pipe? E°Fe = -0.44 V; all E° values refer to the M2+/M half-cell reactions.
A) copper, Cu, E° = 0.15 V
B) cobalt, Co, E° = -0.28 V
C) chromium, Cr, E° = -0.74 V
D) tin, Sn, E° = -0.14 V
E) None of these metals would be capable of acting as a sacrificial anode with iron.
A) copper, Cu, E° = 0.15 V
B) cobalt, Co, E° = -0.28 V
C) chromium, Cr, E° = -0.74 V
D) tin, Sn, E° = -0.14 V
E) None of these metals would be capable of acting as a sacrificial anode with iron.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
62
Which one of the following statements relating to the glass electrode is correct?
A) The glass electrode detects hydrogen gas.
B) The glass of a glass electrode serves to conduct electrons.
C) When pH is measured, only a single electrode, the glass electrode, need be used.
D) The potential of the glass electrode varies linearly with the pH of the solution.
E) None of these choices are correct.
A) The glass electrode detects hydrogen gas.
B) The glass of a glass electrode serves to conduct electrons.
C) When pH is measured, only a single electrode, the glass electrode, need be used.
D) The potential of the glass electrode varies linearly with the pH of the solution.
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following elements could be prepared by electrolysis of the aqueous solution shown?
A) sodium from Na3PO4(aq)
B) sulfur from K2SO4(aq)
C) oxygen from H2SO4(aq)
D) potassium from KCl(aq)
E) nitrogen from AgNO3(aq)
A) sodium from Na3PO4(aq)
B) sulfur from K2SO4(aq)
C) oxygen from H2SO4(aq)
D) potassium from KCl(aq)
E) nitrogen from AgNO3(aq)

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
64
What mass of copper will be deposited when 18.2 A are passed through a CuSO4 solution for 45.0 minutes?
A) 16.2 g
B) 33.4 g
C) 40.6 g
D) 81.3 g
E) 163 g
A) 16.2 g
B) 33.4 g
C) 40.6 g
D) 81.3 g
E) 163 g

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
65
Predict the products of the cell reaction when a molten salt mixture of sodium bromide and calcium fluoride is electrolyzed (spectator ions are not considered to be products).
A) calcium and bromine
B) sodium and fluorine
C) calcium bromide
D) calcium and fluorine
E) sodium and bromine
A) calcium and bromine
B) sodium and fluorine
C) calcium bromide
D) calcium and fluorine
E) sodium and bromine

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
66
A solution is prepared by dissolving 32.0 g of NiSO4 in water. What current would be needed to deposit all of the nickel in 5.0 hours?
A) 1.1 A
B) 2.2 A
C) 3.3 A
D) 4.4 A
E) 5.5 A
A) 1.1 A
B) 2.2 A
C) 3.3 A
D) 4.4 A
E) 5.5 A

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
67
Electrolytic cells utilize electrical energy to drive nonspontaneous redox reactions.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
68
Chromium metal is electroplated from acidic aqueous solutions containing the dichromate ion, Cr2O72-. What is the minimum time needed to plate out 10.0 g of chromium metal from such a solution, if the current is 50.0 A?
A) 6.2 minutes
B) 12.4 minutes
C) 18.6 minutes
D) 24.7 minutes
E) 37.1 minutes
A) 6.2 minutes
B) 12.4 minutes
C) 18.6 minutes
D) 24.7 minutes
E) 37.1 minutes

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
69
What product forms at the cathode during the electrolysis of molten lithium iodide?
A) Li+(l)
B) Li(l)
C) I-(l)
D) I2(g)
E) I3-(l)
A) Li+(l)
B) Li(l)
C) I-(l)
D) I2(g)
E) I3-(l)

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
70
A current of 250. A flows for 24.0 hours at an anode where the reaction occurring is Mn2+(aq) + 2H2O(l) → MnO2(s) + 4H+(aq) + 2e- What mass of MnO2 is deposited at this anode?
A) 19.5 kg
B) 12.9 kg
C) 4.87 kg
D) 2.43 kg
E) None of these choices are correct.
A) 19.5 kg
B) 12.9 kg
C) 4.87 kg
D) 2.43 kg
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
71
In the electrolysis of aqueous potassium nitrate using inert electrodes, which one of the following species is oxidized?
A) potassium ion
B) nitrate ion
C) water
D) oxygen
E) hydronium ion
A) potassium ion
B) nitrate ion
C) water
D) oxygen
E) hydronium ion

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following elements can be isolated by electrolysis of the aqueous salt shown?
A) phosphorus from K3PO4(aq)
B) sodium from NaBr(aq)
C) aluminum from AlCl3(aq)
D) fluorine from KF(aq)
E) iodine from NaI(aq)
A) phosphorus from K3PO4(aq)
B) sodium from NaBr(aq)
C) aluminum from AlCl3(aq)
D) fluorine from KF(aq)
E) iodine from NaI(aq)

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
73
Which, if any, of the following metals would not be capable of acting as a sacrificial anode when used with iron E°Fe = -0.44 V; all E° values refer to the M2+/M half-cell reactions.
A) manganese, Mn, E° = -1.18 V
B) cadmium, Cd, E° = -0.40 V
C) magnesium, Mg, E° = -2.37 V
D) zinc, Zn, E° = -0.76 V
E) All of these metals are capable of acting as sacrificial anodes with iron.
A) manganese, Mn, E° = -1.18 V
B) cadmium, Cd, E° = -0.40 V
C) magnesium, Mg, E° = -2.37 V
D) zinc, Zn, E° = -0.76 V
E) All of these metals are capable of acting as sacrificial anodes with iron.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
74
A battery that cannot be recharged is a
A) fuel cell.
B) primary battery.
C) secondary battery.
D) simple battery.
E) flow battery.
A) fuel cell.
B) primary battery.
C) secondary battery.
D) simple battery.
E) flow battery.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
75
What product forms at the anode during the electrolysis of molten NaBr?
A) Na+(l)
B) Na(l)
C) Br-(l)
D) Br3-(l)
E) Br2(g)
A) Na+(l)
B) Na(l)
C) Br-(l)
D) Br3-(l)
E) Br2(g)

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
76
A concentration cell consists of two Al/Al3+electrodes. The electrolyte in compartment A is 0.050 M Al(NO3)3 and in compartment B is 1.25 M Al(NO3)3. What is the voltage of the cell at 25°C?
A) 0.083 V
B) 0.062 V
C) 0.041 V
D) 0.028 V
E) None of these choices are correct.
A) 0.083 V
B) 0.062 V
C) 0.041 V
D) 0.028 V
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
77
In the electrolysis of aqueous sodium sulfate at electrodes of platinum, predict the products of the cell reaction.
A) sodium and sulfur
B) hydrogen and sulfur
C) oxygen and sulfur
D) oxygen and sulfuric acid
E) hydrogen and oxygen
A) sodium and sulfur
B) hydrogen and sulfur
C) oxygen and sulfur
D) oxygen and sulfuric acid
E) hydrogen and oxygen

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
78
Two cells are connected in series, so that the same current flows through two electrodes where the following half-reactions occur Cu2+(aq) + 2e- → Cu(s) and Ag+(aq) + e- → Ag(s)
For every 1.00 g of copper produced in the first process, how many grams of silver will be
Produced in the second one?
A) 0.294 g
B) 0.588 g
C) 0.850 g
D) 1.70 g
E) 3.40 g
For every 1.00 g of copper produced in the first process, how many grams of silver will be
Produced in the second one?
A) 0.294 g
B) 0.588 g
C) 0.850 g
D) 1.70 g
E) 3.40 g

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
79
How many grams of oxygen gas will be produced in the electrolysis of water, for every gram of hydrogen gas formed? Reaction: 2H2O(l) → 2H2(g) + O2(g)
A) 31.7 g
B) 15.9 g
C) 7.94 g
D) 3.97 g
E) 1.98 g
A) 31.7 g
B) 15.9 g
C) 7.94 g
D) 3.97 g
E) 1.98 g

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
80
What mass of silver will be formed when 15.0 A are passed through molten AgCl for 25.0 minutes?
A) 0.419 g
B) 6.29 g
C) 12.6 g
D) 25.2 g
E) 33.4 g
A) 0.419 g
B) 6.29 g
C) 12.6 g
D) 25.2 g
E) 33.4 g

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck



