Deck 25: The Chemistry of Life: Organic and Biological Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/115
Play
Full screen (f)
Deck 25: The Chemistry of Life: Organic and Biological Chemistry
1
What type of compound has been used to replace tetraethyl lead ((C2H5)4Pb) as an antiknock agent in gasoline?
A) fluorochlorocarbons
B) oxygenated hydrocarbons
C) aromatic compounds
D) olefins
E) paraffins
A) fluorochlorocarbons
B) oxygenated hydrocarbons
C) aromatic compounds
D) olefins
E) paraffins
oxygenated hydrocarbons
2
Which statement about addition reactions between alkenes and HBr is false?
A) Bromine attacks the alkene carbon atom possessing a positive partial charge.
B) The addition occurs at the double bond.
C) The n bond breaks in the course of the reaction.
D) A hydrogen atom attaches itself to the alkene carbon atom possessing a negative partial charge.
E) The proposed mechanism involves radicals.
A) Bromine attacks the alkene carbon atom possessing a positive partial charge.
B) The addition occurs at the double bond.
C) The n bond breaks in the course of the reaction.
D) A hydrogen atom attaches itself to the alkene carbon atom possessing a negative partial charge.
E) The proposed mechanism involves radicals.
The proposed mechanism involves radicals.
3
Proteins are biopolymers formed via multiple condensation coupling of which two functional groups?
A) ester and carboxylic acid
B) amine and carboxylic acid
C) ester and amine
D) alcohol and amine
E) alcohol and carboxylic acid
A) ester and carboxylic acid
B) amine and carboxylic acid
C) ester and amine
D) alcohol and amine
E) alcohol and carboxylic acid
amine and carboxylic acid
4
What is the general formula for a ketone?
A) R-OH
B) R-O-R
C) R-CO-OH
D) R- CO- R'
E) R-CHO
A) R-OH
B) R-O-R
C) R-CO-OH
D) R- CO- R'
E) R-CHO

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
5
Which structure below represents a ketone?
A)
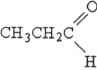
B)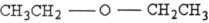
C)
D)
E)
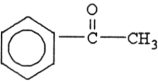
A)

B)
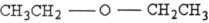
C)

D)

E)


Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
6
Consider the following types of compounds:
(i) amino acid
(ii) nitrogen- containing organic base
(iii) phosphoric acid
(iv) five- carbon sugar
Which of the above compounds are the monomers of nucleic acids, called nucleotides?
A) none
B) (i) and (ii)
C) (ii) and (iv)
D) (ii), (iii), and (iv)
E) all
(i) amino acid
(ii) nitrogen- containing organic base
(iii) phosphoric acid
(iv) five- carbon sugar
Which of the above compounds are the monomers of nucleic acids, called nucleotides?
A) none
B) (i) and (ii)
C) (ii) and (iv)
D) (ii), (iii), and (iv)
E) all

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
7
Which one of the following molecules is chiral?
A)
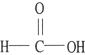
B)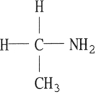
C)
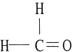
D)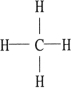
E)
A)
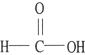
B)

C)
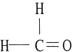
D)

E)


Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
8
How many isomers are possible for C4H10?
A) 1
B) 2
C) 3
D) 4
E) 10
A) 1
B) 2
C) 3
D) 4
E) 10

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
9
The structure of 2,3- dimethylheptane is .
A)
B)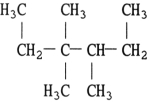
C)
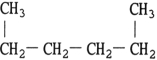
D)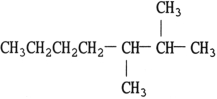
E)
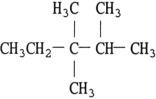
A)

B)

C)
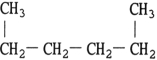
D)

E)


Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
10
Alcohols are hydrocarbon derivatives in which one or more hydrogens have been replaced by a hydroxyl functional group. is the general formula of an alcohol.
A) R-OH
B) R-CO-H
C) R-CO-R
D) R-O-R
E) R-CO-OH
A) R-OH
B) R-CO-H
C) R-CO-R
D) R-O-R
E) R-CO-OH

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
11
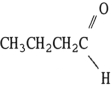
Which one of the following compounds is an isomer of CH3CH2CH2CH2OH?
A)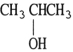
B)
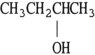
C) CH3OH
D) CH3CH2CH2OH
E)
A)
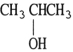
B)
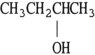
C) CH3OH
D) CH3CH2CH2OH
E)


Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
12
Of the compounds below, is an isomer of
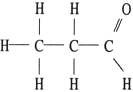
A)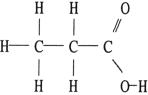
B)
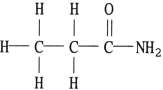
C)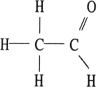
D)
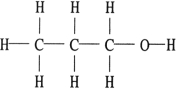
E)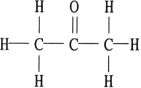

A)
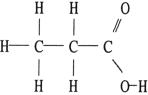
B)
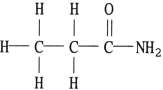
C)

D)

E)
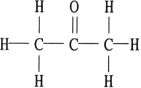

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
13
How many isomers are possible for C5H12?
A) 1
B) 2
C) 3
D) 4
E) 10
A) 1
B) 2
C) 3
D) 4
E) 10

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
14
Consider the following statements about alcohols:
(i) Alcohols contain a polar O- H bond and hence mix well with polar solvents like water.
(ii) Alcohols form hydrogen bonds with water.
(iii) Alcohols have a higher boiling point compared to hydrocarbons with the same number of carbon atoms.
(iv) For the most part, alcohols are toxic.
Which statement(s) is(are) true?
A) none
B) (i) and (ii)
C) (iii) only
D) (i), (ii), and (iv)
E) all
(i) Alcohols contain a polar O- H bond and hence mix well with polar solvents like water.
(ii) Alcohols form hydrogen bonds with water.
(iii) Alcohols have a higher boiling point compared to hydrocarbons with the same number of carbon atoms.
(iv) For the most part, alcohols are toxic.
Which statement(s) is(are) true?
A) none
B) (i) and (ii)
C) (iii) only
D) (i), (ii), and (iv)
E) all

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
15
Sugars are examples of what type of molecule?
A) amino acids
B) salts
C) nucleic acids
D) carbohydrates
E) proteins
A) amino acids
B) salts
C) nucleic acids
D) carbohydrates
E) proteins

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
16
is a monosaccharide.
A) Maltose
B) Fructose
C) Glucose
D) Sucrose
E) Lactose
A) Maltose
B) Fructose
C) Glucose
D) Sucrose
E) Lactose

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
17
When petroleum is distilled to separate the components by boiling point, the component with the highest boiling point is called .
A) gas
B) gasoline
C) paraffin
D) kerosene
E) asphalt
A) gas
B) gasoline
C) paraffin
D) kerosene
E) asphalt

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
18
How many structural isomers (include all types except optical) can be drawn for C5H10?
A) 5
B) 6
C) 7
D) 11
E) 12
A) 5
B) 6
C) 7
D) 11
E) 12

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
19
How many isomers of C2H2Cl2 are polar?
A) none
B) 1
C) 2
D) 3
E) It is impossible to tell without more information.
A) none
B) 1
C) 2
D) 3
E) It is impossible to tell without more information.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
20
During World War II, Teflon™ was used _ .
A) to package the shrapnel incorporated into the atomic bomb
B) as a liner inside the atomic bomb to protect the guidance system from radiation
C) as gasket material in the gaseous diffusion plant for separation of uranium isotopes
D) to form the tube to keep the separate parts of the critical mass apart until the time for detonation of the bomb
E) because its characteristic color change upon exposure to radiation made it an excellent indicator of radiation leaks
A) to package the shrapnel incorporated into the atomic bomb
B) as a liner inside the atomic bomb to protect the guidance system from radiation
C) as gasket material in the gaseous diffusion plant for separation of uranium isotopes
D) to form the tube to keep the separate parts of the critical mass apart until the time for detonation of the bomb
E) because its characteristic color change upon exposure to radiation made it an excellent indicator of radiation leaks

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
21
In DNA adenine is always paired with

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
22
The aromas of different fruit are due to the chemical compounds known as

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following contains a peptide linkage?
A)
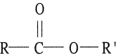
B)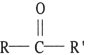
C)
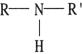
D)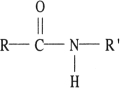
E) none of the above
A)
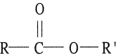
B)
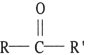
C)
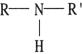
D)
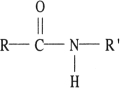
E) none of the above

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
24
The principal difference between fructose and glucose is that .
A) fructose is a disaccharide and glucose is a monosaccharide
B) fructose is a monosaccharide and glucose is a disaccharide
C) fructose is chiral and glucose is not
D) fructose is a ketone sugar and glucose is an aldehyde sugar
E) glucose is chiral and fructose is not
A) fructose is a disaccharide and glucose is a monosaccharide
B) fructose is a monosaccharide and glucose is a disaccharide
C) fructose is chiral and glucose is not
D) fructose is a ketone sugar and glucose is an aldehyde sugar
E) glucose is chiral and fructose is not

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
25
In the reaction of nitric acid with benzene, which isomer is formed when a second nitro group is substituted ?

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following compounds do not contain an sp3 hybridized oxygen atom?
A) alcohols
B) ethers
C) water
D) esters
E) ketones
A) alcohols
B) ethers
C) water
D) esters
E) ketones

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
27
Which one of the following is not an alcohol?
A) cholesterol
B) acetone
C) ethanol
D) glycerol
E) ethylene glycol
A) cholesterol
B) acetone
C) ethanol
D) glycerol
E) ethylene glycol

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
28
Which structure below represents an aldehyde?
A)
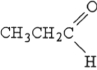
B)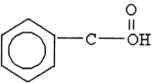
C)
D)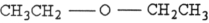
E)
A)

B)

C)

D)
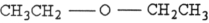
E)


Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
29
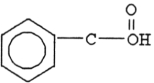
What functional group is characteristic of carboxylic acids?

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following compounds does not contain a C=O bond?
A) ketones
B) ethers
C) aldehydes
D) esters
E) amides
A) ketones
B) ethers
C) aldehydes
D) esters
E) amides

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
31
Rotation around a carbon- carbon double bond is difficult, requiring energy but it is a key process in the chemistry of .

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
32
Which one of the following is a monosaccharide?
A) lactose
B) fructose
C) maltose
D) sucrose
E) none of the above
A) lactose
B) fructose
C) maltose
D) sucrose
E) none of the above

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
33
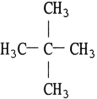
How many chiral carbon atoms does the neopentane (2, 2 - dimethylpropane) have?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
34
If each of the following represents an alkane, and a carbon atom is located at each vertex with the proper number of hydrogen atoms also bonded to it, which one is the most reactive?
A)
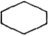
B)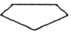
C)

D)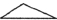
E) They are all equally reactive since they are all alkanes.
A)

B)

C)

D)

E) They are all equally reactive since they are all alkanes.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
35
What is the name of the compound below?
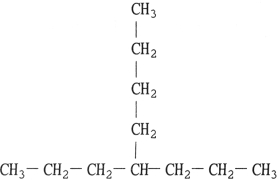


Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
36
Which one of the following could be a cyclic alkane?
A) C2H6
B) C9H20
C) C3H6
D) C5H5
E) C4H6
A) C2H6
B) C9H20
C) C3H6
D) C5H5
E) C4H6

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
37
The addition of an alkyl halide to an aromatic ring compound is called the _ reaction.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
38
Which structure below represents an ether?
A)
B)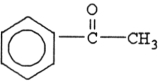
C)
D)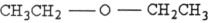
E)
A)

B)

C)

D)
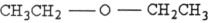
E)


Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
39
Which statement about hydrocarbons is false?
A) The smallest alkane to have structural (constitutional) isomers has 4 carbon atoms.
B) Alkanes can be produced by hydrogenating alkenes.
C) Alkanes are more reactive than alkenes.
D) Alkenes can be polymerized.
E) Cyclic alkanes are structural isomers of alkenes.
A) The smallest alkane to have structural (constitutional) isomers has 4 carbon atoms.
B) Alkanes can be produced by hydrogenating alkenes.
C) Alkanes are more reactive than alkenes.
D) Alkenes can be polymerized.
E) Cyclic alkanes are structural isomers of alkenes.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
40
Benzene behaves differently from a hydrocarbon which simply contains three C=C bonds in that the latter would be expected to react much more readily with .
A) H2
B) Br2
C) HCl
D) Cl2
E) all of the above
A) H2
B) Br2
C) HCl
D) Cl2
E) all of the above

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
41
In the oxidation of ethanol the intermediate formes is .

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
42
What is the correct name for the compound, CH3CH2CH=CHCH2CH=CHCH?

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
43
Predict the product of the catalytic hydrogenation of 6- ethyl- 3- decene.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
44
The anaerobic conversion of carbohydrates to ethanol is driven by the presence of
.
.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
45
Hydrogenation of an alkene requires high temperatures and a catalyst such as nickel. Why is this?

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
46
The resistance of gasoline to engine knocking is referred to as its .

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
47
The hydrolysis of an ester in the presence of a base is called .

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
48
The monomers of nucleic acids, called nucleotides, consist of three parts. These are
.
.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
49
Large protein molecules that act as catalysts are called .

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
50
Write the formula for 2- methyl- 4- propylnonane.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
51
The primary ingredient in vinegar is _

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
52
Electron pairs in alkanes are in a arrangement.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
53
In nature, - amino acids dominate.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
54
Why is cyclopropane more reactive than propane?

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
55
Of the 20 amino acids found in our bodies, of them must be ingested because our bodies cannot synthesize sufficient quantities of them.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
56
Hydrogenation of what alkyne produces propane?

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
57
What is the name of the compound below?
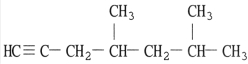


Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
58
Lactose is a disaccharide of glucose and .

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
59
The doubly ionized form of an amino acid is called a _

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
60
Mirror- image isomers of a substance are called _.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
61
The general formula for an ether is .
A) R-CO-OH
B) R-O-R'
C) R-CO-R'
D) R-CO-H
E) R-OH
A) R-CO-OH
B) R-O-R'
C) R-CO-R'
D) R-CO-H
E) R-OH

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
62
The minimum number of carbons necessary for a hydrocarbon to form a branched structure is
)
A) 9
B) 4
C) 3
D) 12
E) 6
)
A) 9
B) 4
C) 3
D) 12
E) 6

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
63
The condensation reaction of a carboxyl group of one amino acid and the amino group of a second amino acid results in the formation of a

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
64
Which substance would be the most soluble in gasoline _?
A) NaNO3
B) HCl
C) NaCl
D) hexane
E) water
A) NaNO3
B) HCl
C) NaCl
D) hexane
E) water

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
65
In general, are the most reactive hydrocarbons.
A) alkenes
B) alkynes
C) olefins
D) cycloalkanes
E) alkanes
A) alkenes
B) alkynes
C) olefins
D) cycloalkanes
E) alkanes

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
66
The general formula of an ester is _.
A) R-O-R'
B) R-CO-OH
C) R-CO-R'
D) R-OH
E) R-CO-OR'
A) R-O-R'
B) R-CO-OH
C) R-CO-R'
D) R-OH
E) R-CO-OR'

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
67
How many structural isomers of heptane exist ?
A) 2
B) 4
C) 6
D) 8
E) 10
A) 2
B) 4
C) 6
D) 8
E) 10

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
68
Alkenes always contain a _ .
A) C- C bond
B) C=C bond
C) C≡H bond
D) C=H bond
E) C≡C bond
A) C- C bond
B) C=C bond
C) C≡H bond
D) C=H bond
E) C≡C bond

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
69
CH3CH2C(=O)NH2 is called a(n) .
A) ester
B) aldehyde
C) amine
D) amide
E) ketone
A) ester
B) aldehyde
C) amine
D) amide
E) ketone

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
70
Hydrocarbons containing only single bonds between the carbon atoms are called _.
A) aromatics
B) alkanes
C) ketones
D) alkynes
E) alkenes
A) aromatics
B) alkanes
C) ketones
D) alkynes
E) alkenes

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
71
The octane number of straight- run gasoline is about _ _.
A) 50
B) 0
C) 93
D) 75
E) 25
A) 50
B) 0
C) 93
D) 75
E) 25

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
72
Alkynes always contain a _.
A) C=C bond
B) C≡H bond
C) C-C bond
D) C=H bond
E) C≡C bond
A) C=C bond
B) C≡H bond
C) C-C bond
D) C=H bond
E) C≡C bond

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
73
Cyclohexane has fewer hydrogens than n- hexane.
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
74
Alkenes have the general formula _.
A) C2nHn.
B) CnHn.
C) CnH2n+2.
D) CnH2n- 2.
E) CnH2n.
A) C2nHn.
B) CnHn.
C) CnH2n+2.
D) CnH2n- 2.
E) CnH2n.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
75
What is the name of the compound below _ _?
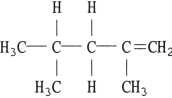
A) 2,4- ethylbutene
B) 2,5- dimethylpentane
C) 2,4- dimethyl- 1- pentene
D) 2,4- methylbutene
E) 2,4- dimethyl- 4- pentene
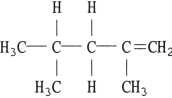
A) 2,4- ethylbutene
B) 2,5- dimethylpentane
C) 2,4- dimethyl- 1- pentene
D) 2,4- methylbutene
E) 2,4- dimethyl- 4- pentene

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
76
Gasoline and water do not mix because gasoline is .
A) less dense than water
B) polar and water is nonpolar
C) nonpolar and water is polar
D) less viscous than water
E) volatile and water is not
A) less dense than water
B) polar and water is nonpolar
C) nonpolar and water is polar
D) less viscous than water
E) volatile and water is not

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
77
What is the name of the compound CH3CH2CH(OH)CH2CH2CH3?

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
78
Aromatic hydrocarbons _ _.
A) undergo substitution reactions more easily than saturated hydrocarbons
B) are stabilized by resonance
C) have sp2 hybridized carbon atoms
D) contain a series of n bonds on several consecutive carbon atoms
E) readily undergo addition reactions like alkenes
A) undergo substitution reactions more easily than saturated hydrocarbons
B) are stabilized by resonance
C) have sp2 hybridized carbon atoms
D) contain a series of n bonds on several consecutive carbon atoms
E) readily undergo addition reactions like alkenes

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
79
Optically active molecules that are mirror images of each other are called .
A) enantiomers
B) geometrical isomers
C) cofactors
D) allotropes
E) chiral compounds
A) enantiomers
B) geometrical isomers
C) cofactors
D) allotropes
E) chiral compounds

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
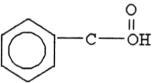
80
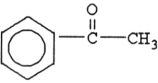
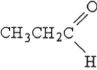
The compound below is a(n) .
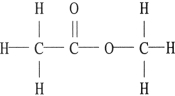
A) carboxylic acid
B) amine
C) ketone
D) aldehyde
E) ester
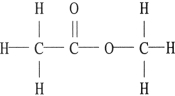
A) carboxylic acid
B) amine
C) ketone
D) aldehyde
E) ester

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck



