Deck 14: Mass Spectrometry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/75
Play
Full screen (f)
Deck 14: Mass Spectrometry
1
What is the x-axis of a mass spectrum?
A) mass
B) mass/energy
C) mass/charge
D) charge
A) mass
B) mass/energy
C) mass/charge
D) charge
mass/charge
2
What are the most common products of the fragmentation of a molecular ion?
A) two radicals
B) an anion and a cation
C) a radical and a cation
D) two cations
A) two radicals
B) an anion and a cation
C) a radical and a cation
D) two cations
a radical and a cation
3
Which of the following is most consistent with a molecule having a molecular ion with m/e of 77?
A) the molecule contains bromine
B) the molecule contains nitrogen
C) the molecule is an alcohol
D) the molecule is a hydrocarbon
A) the molecule contains bromine
B) the molecule contains nitrogen
C) the molecule is an alcohol
D) the molecule is a hydrocarbon
the molecule contains nitrogen
4
What is the y-axis of a mass spectrum?
A) mass
B) energy
C) abundance
D) field strength
A) mass
B) energy
C) abundance
D) field strength

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the molecules gives rise to a molecular ion with an odd value of m/z?
A) C6H12O2
B) C7H10ClN
C) C8H18N2
D) C8H12
A) C6H12O2
B) C7H10ClN
C) C8H18N2
D) C8H12

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following sets of molecular ions does an alkyl chloride possess?
A) two molecular ions in a 2:1 ratio separated by two mass units
B) two molecular ions in a 1:1 ratio separated by two mass units
C) three molecular ions in a 1:2:1 ratio separated by two mass units
D) two molecular ions in a 3:1 ratio separated by two mass units
A) two molecular ions in a 2:1 ratio separated by two mass units
B) two molecular ions in a 1:1 ratio separated by two mass units
C) three molecular ions in a 1:2:1 ratio separated by two mass units
D) two molecular ions in a 3:1 ratio separated by two mass units

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
7
How many carbon atoms are present in a molecule which gives an M+1 peak that is 5.74% of the intensity of the molecular ion?
A) 5
B) 6
C) 11
D) 12
A) 5
B) 6
C) 11
D) 12

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the molecules gives rise to a molecular ion with an odd value of m/z?
A) C6H12Cl2
B) C7H10N2
C) C8H13N
D) C8H14Br2
A) C6H12Cl2
B) C7H10N2
C) C8H13N
D) C8H14Br2

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
9
What is the principle that allows us to use mass spectrometry to determine the molecular weight of a compound?
A) higher molecular weight compounds are less volatile
B) higher molecular weight compounds are more dense
C) a beam of higher molecular weight cations is deflected less by a magnetic field
D) ions with higher molecular weight absorb higher frequencies of light
A) higher molecular weight compounds are less volatile
B) higher molecular weight compounds are more dense
C) a beam of higher molecular weight cations is deflected less by a magnetic field
D) ions with higher molecular weight absorb higher frequencies of light

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is not prominent in the mass spectrum of 2-methyl-2-butanol?
A) M − 29
B) M − 18
C) M − 15
D) M − 12
A) M − 29
B) M − 18
C) M − 15
D) M − 12

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
11
What are products of the collision between high energy electrons and methane?
A) CH4− ⋅ + 2 e−
B) CH3− + H⋅
C) CH4+⋅ + 2 e−
D) CH3⋅ + H+ + 2 e−
A) CH4− ⋅ + 2 e−
B) CH3− + H⋅
C) CH4+⋅ + 2 e−
D) CH3⋅ + H+ + 2 e−

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
12
In mass spectrometry, which of the following phrases is shortened to the acronym "FAB"?
A) flight averaged bumping
B) fast atom bombardment
C) fragmented atom beam
D) flying atom bashing
A) flight averaged bumping
B) fast atom bombardment
C) fragmented atom beam
D) flying atom bashing

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following bonds of the octane radical cation is least subject to fragmentation?
i ii iii iv
A) i
B) ii
C) iii
D) iv
i ii iii iv
A) i
B) ii
C) iii
D) iv

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following corresponds to the molecular ion in EI mass spectrometry?
A) the most abundant ion
B) the ion with the smallest m/e
C) the ion formed by removal of an electron from the molecule
D) any ion formed by fragmentation of the molecule
A) the most abundant ion
B) the ion with the smallest m/e
C) the ion formed by removal of an electron from the molecule
D) any ion formed by fragmentation of the molecule

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following sets of molecular ions does a dibromoalkane possess?
A) two molecular ions in a 2:1 ratio separated by two mass units
B) two molecular ions in a 1:1 ratio separated by two mass units
C) three molecular ions in a 1:2:1 ratio, separated by two mass units
D) two molecular ions in a 3:1 ratio separated by two mass units
A) two molecular ions in a 2:1 ratio separated by two mass units
B) two molecular ions in a 1:1 ratio separated by two mass units
C) three molecular ions in a 1:2:1 ratio, separated by two mass units
D) two molecular ions in a 3:1 ratio separated by two mass units

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following sets of molecular ions does an alkyl bromide possess?
A) two molecular ions in a 2:1 ratio separated by two mass units
B) two molecular ions in a 1:1 ratio separated by two mass units
C) three molecular ions in a 1:2:1 ratio separated by two mass units
D) two molecular ions in a 3:1 ratio separated by two mass units
A) two molecular ions in a 2:1 ratio separated by two mass units
B) two molecular ions in a 1:1 ratio separated by two mass units
C) three molecular ions in a 1:2:1 ratio separated by two mass units
D) two molecular ions in a 3:1 ratio separated by two mass units

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
17
In mass spectrometry, which of the following phrases is shortened to the acronym "EI"?
A) electron injection
B) electron impact
C) energy initiated
D) energy insertion
A) electron injection
B) electron impact
C) energy initiated
D) energy insertion

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
18
How many carbon atoms are present in a molecule which gives an M+1 peak that is 11.48% of the intensity of the molecular ion?
A) 5
B) 6
C) 11
D) 12
A) 5
B) 6
C) 11
D) 12

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
19
In mass spectrometry, which of the following phrases is shortened to the acronym "MALDI"?
A) mass analysis by light-detected ions
B) matrix assisted laser desorption ionization
C) many atom loss-detecting ionization
D) molecular and light detecting instrument
A) mass analysis by light-detected ions
B) matrix assisted laser desorption ionization
C) many atom loss-detecting ionization
D) molecular and light detecting instrument

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following is the definition of the base peak of a mass spectrum?
A) the peak corresponding to the most abundant ion
B) the peak corresponding to the ion with lowest m/e
C) the peak corresponding to the molecular ion peak
D) the peak corresponding to the ion arising to loss of a proton from the molecular ion
A) the peak corresponding to the most abundant ion
B) the peak corresponding to the ion with lowest m/e
C) the peak corresponding to the molecular ion peak
D) the peak corresponding to the ion arising to loss of a proton from the molecular ion

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following is not prominent in the mass spectrum of 2-methyl-2-butanol?
A) M − 15
B) M − 18
C) M − 21
D) M − 29
A) M − 15
B) M − 18
C) M − 21
D) M − 29

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following gives rise to a prominent peak in the mass spectrum at m/z of 39?
A) 1-octyne
B) 4-octyne
C) 5-methyl-3-octyne
D) 2,2-dimethyl-3-heptyne
A) 1-octyne
B) 4-octyne
C) 5-methyl-3-octyne
D) 2,2-dimethyl-3-heptyne

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following gives rise to a prominent peak in the mass spectrum with m/z of 30?
A) 1-hexanamine, CH3CH2CH2CH2CH2CH2NH2
B) 1,1-dimethyl-1-butanamine, CH3CH2CH2C(CH3)2NH2
C) 1-hexanol
D) hexanal
A) 1-hexanamine, CH3CH2CH2CH2CH2CH2NH2
B) 1,1-dimethyl-1-butanamine, CH3CH2CH2C(CH3)2NH2
C) 1-hexanol
D) hexanal

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
24
C6H15N could have a mass spectrum with M+ at m/z = 101 with a minor M+1 peak.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following gives rise to a prominent peak in the mass spectrum with m/z of 60?
A) 1-hexanol
B) 2-hexanol
C) hexanal
D) hexanoic acid
A) 1-hexanol
B) 2-hexanol
C) hexanal
D) hexanoic acid

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following gives rise to a prominent M − 15 peak in the mass spectrum?
A) hexane
B) 1-chloropentane
C) 1-pentanol
D) 3-methylpentane
A) hexane
B) 1-chloropentane
C) 1-pentanol
D) 3-methylpentane

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
27
It is not possible distinguish 2,2-dimethylbutane from 2-methylpentane using mass spectrometry.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following gives rise to a prominent M − 18 peak in the mass spectrum?
A) hexane
B) 1-chloropentane
C) 1-pentanol
D) 3-methylpentane
A) hexane
B) 1-chloropentane
C) 1-pentanol
D) 3-methylpentane

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following gives rise to a prominent peak in the mass spectrum with m/z of 91?
A) 1-octene
B) 1-hexanamine, CH3CH2CH2CH2CH2CH2NH2
C) benzene
D) ethylbenzene
A) 1-octene
B) 1-hexanamine, CH3CH2CH2CH2CH2CH2NH2
C) benzene
D) ethylbenzene

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following does not give rise to a prominent M − 41 peak in the mass spectrum?
A) 1-hexene
B) 3-methyl-1-hexene
C) 4-methyl-1-hexene
D) 5-methyl-1-hexene
A) 1-hexene
B) 3-methyl-1-hexene
C) 4-methyl-1-hexene
D) 5-methyl-1-hexene

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
31
Both mass spectrometry and infrared spectroscopy involve the interaction of molecules with electromagnetic energy.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following gives rise to a prominent M − 15 peak in the mass spectrum?
A) cyclopentane
B) cyclopentene
C) cyclopentanol
D) methylcyclopentane
A) cyclopentane
B) cyclopentene
C) cyclopentanol
D) methylcyclopentane

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following alcohols does not give a prominent M − 15 ion?
A) isopropyl alcohol
B) tert-butyl alcohol
C) 2-hexanol
D) 3-pentanol
A) isopropyl alcohol
B) tert-butyl alcohol
C) 2-hexanol
D) 3-pentanol

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following gives rise to a prominent peak in the mass spectrum with m/z of 45?
A) 1-hexanol
B) 2-hexanol
C) hexanal
D) hexanoic acid
A) 1-hexanol
B) 2-hexanol
C) hexanal
D) hexanoic acid

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following gives rise to a prominent peak in the mass spectrum with m/z of 43?
A) 1-hexanol
B) 2-hexanol
C) hexanal
D) hexanoic acid
A) 1-hexanol
B) 2-hexanol
C) hexanal
D) hexanoic acid

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following gives rise to a prominent M − 18 peak in the mass spectrum?
A) cyclohexane
B) cyclohexene
C) 1-chlorocyclohexane
D) cyclohexanol
A) cyclohexane
B) cyclohexene
C) 1-chlorocyclohexane
D) cyclohexanol

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
37
Loratidine is the active ingredient in the antihistamine Claritin®. Mass spectral analysis of loratidine shows M+ at m/z = 382 and M+ at m/z = 384 in an approximate ratio of 3:1 in intensity. This data indicates that loratidine contains fluorine.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following gives rise to a prominent peak in the mass spectrum with m/z of 41?
A) 1-hexene
B) 2-hexyne
C) 3-hexanol
D) 2-chlorohexane
A) 1-hexene
B) 2-hexyne
C) 3-hexanol
D) 2-chlorohexane

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
39
Alpha cleavage of the following compound would produce fragments at m/z = 43 and m/z = 119. 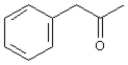


Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following gives rise to a prominent peak in the mass spectrum with m/z of M − 31?
A) 2-methylhexanoic acid
B) methyl hexanoate
C) ethyl hexanoate
D) hexanoic acid
A) 2-methylhexanoic acid
B) methyl hexanoate
C) ethyl hexanoate
D) hexanoic acid

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
41
Refer to the mass spectrum of 2-methylbutane shown below to answer the following question(s). 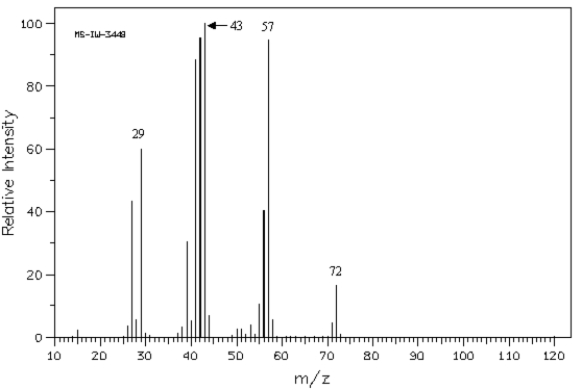 Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
The peak at a m/z of _____ represents the base peak.
 Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/The peak at a m/z of _____ represents the base peak.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
42
Mass spectrometry and infrared spectroscopy are complementary techniques because mass spectrometry provides information about the molar mass and formula while infrared spectroscopy helps identify the functional groups in the formula.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
43
Dehydration of the following compound will produce a peak at a m/z of _______. 


Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
44
Refer to the mass spectrum of 2-methylbutane shown below to answer the following question(s).  Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
The peak at a m/z of _____ represents the following species.
+CH2CH3
 Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/The peak at a m/z of _____ represents the following species.
+CH2CH3

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
45
Cyclohexene and hex-2-yne both have the molecular formula, C6H10. The mass spectrum below belongs to ___________________. 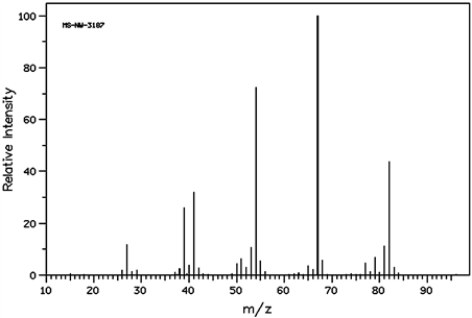


Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
46
Alpha cleavage of the following compound will produce a peak at a m/z of _______. 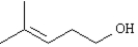


Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
47
What is the most likely molecular formula of a compound (that contains only C, H and O) which has a molecular ion in the EI mass spectrum with m/z of 104 and an M+1 peak that is 5.5% of the intensity of the molecular ion peak?

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
48
Consider the following mass spectrum 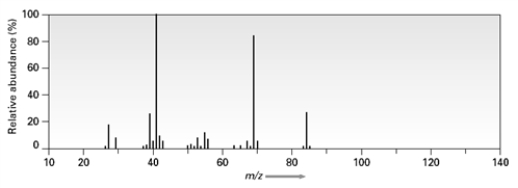 C5H12O might have been used to produce this spectrum.
C5H12O might have been used to produce this spectrum.
 C5H12O might have been used to produce this spectrum.
C5H12O might have been used to produce this spectrum.
Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
49
What is the most likely molecular formula of a compound that has a molecular ion in the EI mass spectrum with m/z of 136, with a M+1 and M+2 peaks that are 4.4% and 49% of the intensity of the molecular ion peak, respectively.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
50
What is the most likely molecular formula of a compound that has a molecular ion in the EI mass spectrum with m/z of 120, with a M+1 and M+2 peaks that are 6.6% and 24% of the intensity of the molecular ion peak, respectively.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
51
A monochloroalkane shows two parent ion peaks m/z at 92 and 94. The molecular formula of this compound might be C3H7Cl.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
52
Refer to the mass spectrum of 2-methylbutane shown below to answer the following question(s).  Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
The peak at a m/z of _____ represents M+.
 Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/The peak at a m/z of _____ represents M+.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
53
Refer to the mass spectrum of 2-methylbutane shown below to answer the following question(s).  Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
The peak at a m/z of _____ represents the following species.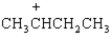
 Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/The peak at a m/z of _____ represents the following species.
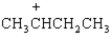

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
54
The following question(s) refer to the mass spectrum shown below. 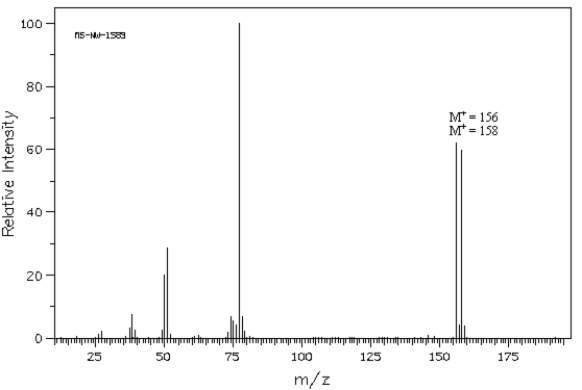 Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
The base peak in this spectrum occurs at a m/z of ______.
 Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/The base peak in this spectrum occurs at a m/z of ______.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
55
The mass of the larger charged fragment produced by the dehydration of the following compound occurs at a m/z of _______. 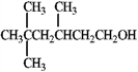
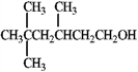

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
56
The mass spectrum of 1-pentanol shows an intense daughter ion peak at m/z = 31. This peak could be due to the formation of CH3O+.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
57
Refer to the mass spectrum of 2-methylbutane shown below to answer the following question(s).  Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
The peak at a m/z of _____ represents the following species.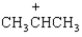
 Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/The peak at a m/z of _____ represents the following species.
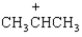

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
58
What is the most likely molecular formula of a compound that has a molecular ion in the EI mass spectrum with m/z of 97 and an M+1 peak that is 6.6% of the intensity of the molecular ion peak?

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
59
Provide the mass of the three most prominent fragment peaks that are observed in the EI mass spectrum of 2-hexanone, CH3CH2CH2CH2COCH3 (molecular ion m/z = 100) and describe the processes that lead to each of these fragments.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
60
This compound contains C, H, and one other atom. The other atom from the mass spectrum is __________.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
61
A mass spectral analysis of 3-methyl-1-pentamine showed a base peak at m/z = 30. The base peak is because of _____.
A)
B)
C)
D)
A)
B)
C)
D)

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
62
Which C6H12O carbonyl-containing compound shows a molecular ion peak at m/z 100 and significant fragment peaks at m/z 85, 58, 57, 43 and 42 in the EI mass spectrum?

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
63
The mass spectral analysis of toluene showed a base peak at m/z = 91. The base peak is because of _____.
A)
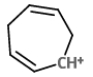
B)
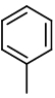
C)
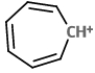
D)
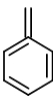
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
64
Match the terms with their descriptions.
Sample is charged by passing it through a charged capillary tube
A)EI-MS
B)FAB
C)MALDI
D)ESI-MS
Sample is charged by passing it through a charged capillary tube
A)EI-MS
B)FAB
C)MALDI
D)ESI-MS

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
65
Mass spectrometry is an analytical technique used to determine the molecular masses, the molecular formulae, and the _____ of compounds.
A) molecular radicals
B) molecular structures
C) functional groups
D) solubilities
A) molecular radicals
B) molecular structures
C) functional groups
D) solubilities

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
66
Identify a basic function of a mass spectrometer.
A) It separates molecules according to their functional groups.
B) It measures only the abundance of isotopes present in molecules.
C) It converts neutral molecules into positively or negatively charged ions.
D) It separates ions according to their molecular masses.
A) It separates molecules according to their functional groups.
B) It measures only the abundance of isotopes present in molecules.
C) It converts neutral molecules into positively or negatively charged ions.
D) It separates ions according to their molecular masses.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
67
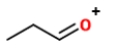
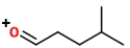
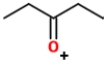
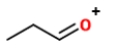
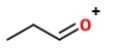
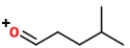
Identify the ions "X" and "Y" in the given reaction. 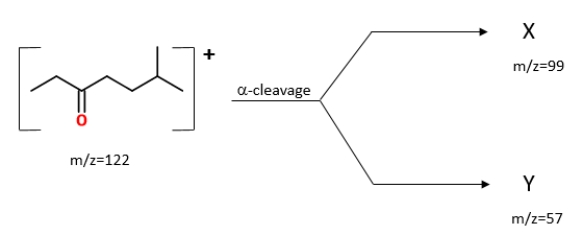
A) X is
 , and Y is
, and Y is
 .
.
B) X is
 , and Y is
, and Y is
 .
.
C) X is
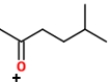 , and Y is
, and Y is
 .
.
D) X is
 , and Y is
, and Y is
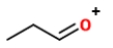 .
.

A) X is
 , and Y is
, and Y is .
.B) X is
 , and Y is
, and Y is .
.C) X is
 , and Y is
, and Y is .
.D) X is
 , and Y is
, and Y is .
.
Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
68
Provide the mass of the three most prominent fragment peaks that are observed in the EI mass spectrum of methyl pentanoate, CH3CH2CH2CH2CO2CH3 (molecular ion m/z = 116) and describe the processes that lead to each of these fragments.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
69
Match the terms with their descriptions.
Sample is bombarded with photons obtained from a laser source
A)EI-MS
B)FAB
C)MALDI
D)ESI-MS
Sample is bombarded with photons obtained from a laser source
A)EI-MS
B)FAB
C)MALDI
D)ESI-MS

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
70
Which C5H12O compound give the following EI mass spectrum? 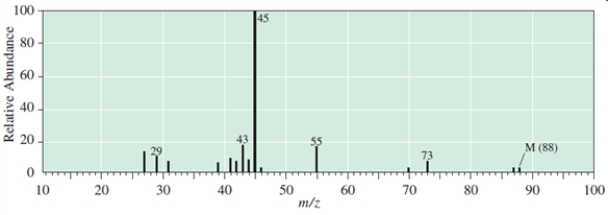


Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
71
Which C6H12O carbonyl-containing compound shows a molecular ion peak at m/z 100 and significant fragment peaks at m/z 71, 58, 57, 43 and 29 in the EI mass spectrum?

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
72
Match the terms with their descriptions.
Sample is bombarded with high-energy particles such as xenon atoms
A)EI-MS
B)FAB
C)MALDI
D)ESI-MS
Sample is bombarded with high-energy particles such as xenon atoms
A)EI-MS
B)FAB
C)MALDI
D)ESI-MS

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
73
Match the terms with their descriptions.
Sample is bombarded with high-energy electrons
A)EI-MS
B)FAB
C)MALDI
D)ESI-MS
Sample is bombarded with high-energy electrons
A)EI-MS
B)FAB
C)MALDI
D)ESI-MS

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
74
Which C5H12O compound give the following EI mass spectrum? 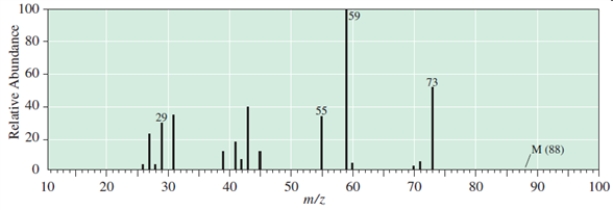


Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
75
Identify the compound(s) that will undergo McLafferty rearrangement during a mass spectral analysis. 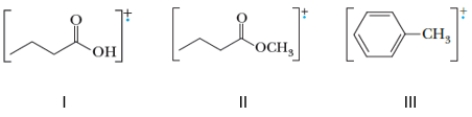
A) Only I
B) Only II
C) Only I and II
D) Only II and III

A) Only I
B) Only II
C) Only I and II
D) Only II and III

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck



