Deck 13: Nuclear Magnetic Resonance Spectroscopy
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/99
Play
Full screen (f)
Deck 13: Nuclear Magnetic Resonance Spectroscopy
1
Which of the following combinations of peaks appears in the 1H NMR spectrum of 1,4-dimethyoxyethane, CH3OCH2CH2CH2CH2OCH3?
A) three singlets
B) a singlet, a triplet, and a quintet
C) a singlet and two triplets
D) a doublet and a triplet
A) three singlets
B) a singlet, a triplet, and a quintet
C) a singlet and two triplets
D) a doublet and a triplet
a singlet and two triplets
2
Which feature in the 1H NMR spectrum provides information about the number of types of different protons in a compound?
A) number of signals
B) integral
C) splitting
D) chemical shift
A) number of signals
B) integral
C) splitting
D) chemical shift
number of signals
3
Which feature in the 1H NMR spectrum provides information about the number of neighboring protons of each proton in the compound?
A) number of signals
B) integral
C) multiplicity
D) chemical shift
A) number of signals
B) integral
C) multiplicity
D) chemical shift
multiplicity
4
Which of the following combinations of peaks appears in the 1H NMR spectrum of 2,3-dimethylbutane?
A) two doublets
B) a doublet and a septet
C) a singlet and two doublets
D) a doublet and an octet
A) two doublets
B) a doublet and a septet
C) a singlet and two doublets
D) a doublet and an octet

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following combinations of peaks appears in the 1H NMR spectrum of butane?
A) a triplet and a doublet
B) a triplet and a quartet
C) a triplet and a sextet
D) two singlets
A) a triplet and a doublet
B) a triplet and a quartet
C) a triplet and a sextet
D) two singlets

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
6
Which C4H9Br compound gives a triplet at approximately 3.5 ppm in the 1H NMR spectrum? 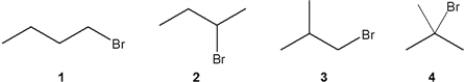
A) 1
B) 2
C) 3
D) 4

A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following combinations of peaks appears in the 1H NMR spectrum of diethyl ether, CH3CH2OCH2CH3?
A) a triplet and a doublet
B) a quartet and a sextet
C) two singlets
D) a triplet and a quartet
A) a triplet and a doublet
B) a quartet and a sextet
C) two singlets
D) a triplet and a quartet

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
8
How many sets of equivalent protons are there in 3-methylhexane?
A) 2
B) 3
C) 6
D) 7
A) 2
B) 3
C) 6
D) 7

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
9
What is the splitting of the signal in the 1H NMR spectrum for the methyl protons of propane?
A) singlet
B) doublet
C) triplet
D) quartet
A) singlet
B) doublet
C) triplet
D) quartet

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is not true regarding 1H NMR spectroscopy?
A) "shielding" leads to peaks at lower values of δ
B) a "downfield" peak appears at a higher value of δ
C) δ for a particular proton depends on the magnetic field strength of the instrument used.
D) on a 300 MHz instrument, a proton that adsorbs irradiation at a frequency 1200 Hz higher than the adsorption of TMS appears at δ 4 ppm.
A) "shielding" leads to peaks at lower values of δ
B) a "downfield" peak appears at a higher value of δ
C) δ for a particular proton depends on the magnetic field strength of the instrument used.
D) on a 300 MHz instrument, a proton that adsorbs irradiation at a frequency 1200 Hz higher than the adsorption of TMS appears at δ 4 ppm.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following combinations of peaks appears in the 1H NMR spectrum of 1,2-dimethyoxyethane, CH3OCH2CH2OCH3?
A) two singlets
B) a singlet and a triplet
C) a singlet and two triplets
D) a doublet and a triplet
A) two singlets
B) a singlet and a triplet
C) a singlet and two triplets
D) a doublet and a triplet

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
12
Which feature in the 1H NMR spectrum provides information about the electronic environment of the protons in a compound?
A) number of signals
B) integral
C) splitting
D) chemical shift
A) number of signals
B) integral
C) splitting
D) chemical shift

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
13
How many sets of equivalent protons are there in 2-methylhexane?
A) 2
B) 3
C) 6
D) 7
A) 2
B) 3
C) 6
D) 7

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
14
What is the appearance of the signal corresponding to the CH2 protons in the 1H NMR spectrum of ethyl methyl ether, CH3CH2OCH3?
A) a doublet
B) a triplet
C) a quartet
D) a septet
A) a doublet
B) a triplet
C) a quartet
D) a septet

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
15
How many sets of equivalent protons are there in hexane?
A) 2
B) 3
C) 6
D) 7
A) 2
B) 3
C) 6
D) 7

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following combinations of peaks appears in the 1H NMR spectrum of 2-methylpropane?
A) two singlets
B) a singlet and a nonet
C) a singlet and a decet
D) a doublet and a decet
A) two singlets
B) a singlet and a nonet
C) a singlet and a decet
D) a doublet and a decet

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
17
What is the splitting of the signal in the 1H NMR spectrum for the methyl protons of ethane?
A) singlet
B) doublet
C) triplet
D) quartet
A) singlet
B) doublet
C) triplet
D) quartet

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is not true regarding 1H NMR spectroscopy?
A) a "downfield" peak appears at a lower value of δ
B) on a 300 MHz instrument, a proton that adsorbs irradiation at a frequency 900 Hz higher than the adsorption of TMS appears at δ 3 ppm.
C) δ for a particular proton is independent of the magnetic field strength of the instrument used.
D) "deshielding" leads to peaks at higher values of δ
A) a "downfield" peak appears at a lower value of δ
B) on a 300 MHz instrument, a proton that adsorbs irradiation at a frequency 900 Hz higher than the adsorption of TMS appears at δ 3 ppm.
C) δ for a particular proton is independent of the magnetic field strength of the instrument used.
D) "deshielding" leads to peaks at higher values of δ

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
19
What is the splitting of the signal in the 1H NMR spectrum for the methyl protons of 1-bromo-2-methylpropane?
A) singlet
B) doublet
C) triplet
D) nonet
A) singlet
B) doublet
C) triplet
D) nonet

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
20
Which feature in the 1H NMR spectrum provides information about the relative number of each type of proton in a compound?
A) number of signals
B) integral
C) splitting
D) chemical shift
A) number of signals
B) integral
C) splitting
D) chemical shift

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
21
How many signals appear in proton-decoupled 13C NMR spectrum of 3,4-dimethylhexane?
A) 3
B) 4
C) 5
D) 6
A) 3
B) 4
C) 5
D) 6

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
22
Which C4H9Br compound(s) gives a 13C NMR spectrum consisting of two signals? 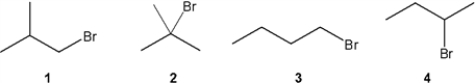
A) only 1
B) only 2
C) only 2 and 3
D) 1, 2, 3 and 4

A) only 1
B) only 2
C) only 2 and 3
D) 1, 2, 3 and 4

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the protons in the following molecule appear furthest downfield in the 1H NMR spectrum? O
||
i ii iii iv
A) i
B) ii
C) iii
D) iv
||
i ii iii iv
A) i
B) ii
C) iii
D) iv

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
24
Which C4H9Br compound(s) gives a 13C NMR spectrum consisting of four signals? 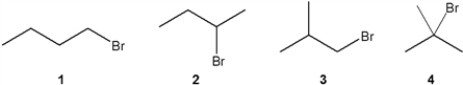
A) only 1
B) only 1 and 2
C) only 2 and 3
D) 1, 2, 3 and 4

A) only 1
B) only 1 and 2
C) only 2 and 3
D) 1, 2, 3 and 4

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
25
How many signals appear in the proton-decoupled 13C NMR spectrum of 1,2-dibromobenzene?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
26
How many signals appear in the proton-decoupled 13C NMR spectrum of 2-bromotoluene?
A) 3
B) 4
C) 5
D) 7
A) 3
B) 4
C) 5
D) 7

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the protons in the following molecule appear furthest downfield in the 1H NMR spectrum?
i ii iii iv
A) i
B) ii
C) iii
D) iv
i ii iii iv
A) i
B) ii
C) iii
D) iv

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the protons in the following molecule appear furthest downfield in the 1H NMR spectrum? O
||
i ii iii iv
A) i
B) ii
C) iii
D) iv
||
i ii iii iv
A) i
B) ii
C) iii
D) iv

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
29
How many signals appear in the proton-decoupled 13C NMR spectrum of 4-bromotoluene?
A) 3
B) 4
C) 5
D) 7
A) 3
B) 4
C) 5
D) 7

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
30
Which C4H9Br compound gives a doublet at approximately 3.3 ppm in the 1H NMR spectrum? 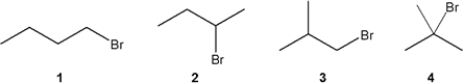
A) 1
B) 2
C) 3
D) 4

A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the protons in the following molecule appear at the highest ?-value in the 1H NMR spectrum? O
||
i ii iii iv
A) i
B) ii
C) iii
D) iv
||
i ii iii iv
A) i
B) ii
C) iii
D) iv

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
32
How many signals appear in proton-decoupled 13C NMR spectrum of 2,5-dimethylhexane?
A) 3
B) 4
C) 5
D) 6
A) 3
B) 4
C) 5
D) 6

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
33
What is the relative area of the six peaks in a septet in an 1H NMR spectrum?
A) 1:2:3:4:3:2:1
B) 1:3:6:12:6:3:1
C) 1:6:15:30:15:6:1
D) 1:6:15:20:15:6:1
A) 1:2:3:4:3:2:1
B) 1:3:6:12:6:3:1
C) 1:6:15:30:15:6:1
D) 1:6:15:20:15:6:1

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
34
How many signals appear in the proton-decoupled 13C NMR spectrum of 3-bromotoluene?
A) 3
B) 4
C) 5
D) 7
A) 3
B) 4
C) 5
D) 7

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the protons in the following molecule appear at the highest δ-value in the 1H NMR spectrum? 
A) i
B) ii
C) iii
D) iv

A) i
B) ii
C) iii
D) iv

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following compounds gives a 1H NMR spectrum consisting of two singlets and a 13C NMR consisting of consisting of three signals? 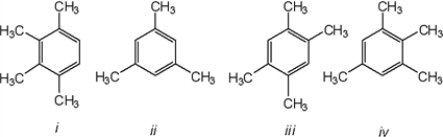
A) only i
B) only iii
C) only ii and iii
D) only ii, iii and iv

A) only i
B) only iii
C) only ii and iii
D) only ii, iii and iv

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
37
What is the relative area of the six peaks in a sexet in an 1H NMR spectrum?
A) 1:2:3:3:2:1
B) 1:3:6:6:3:1
C) 1:5:10:10:5:1
D) 1:6:15:15:6:1
A) 1:2:3:3:2:1
B) 1:3:6:6:3:1
C) 1:5:10:10:5:1
D) 1:6:15:15:6:1

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
38
How many signals appear in the proton-decoupled 13C NMR spectrum of 1,4-dibromobenzene?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the protons in the following molecule appear at the highest δ-value in the 1H NMR spectrum? 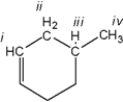
A) i
B) ii
C) iii
D) iv

A) i
B) ii
C) iii
D) iv

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
40
How many signals appear in the proton-decoupled 13C NMR spectrum of 1,3-dibromobenzene?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
41
Which C9H10O compound gives the following 1H NMR spectrum? 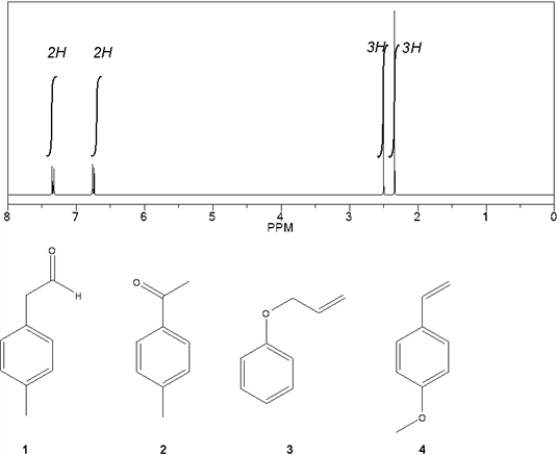
A) 1
B) 2
C) 3
D) 4

A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
42
What is the hydrogen deficiency index for a compound with a molecular formula of C6H11N?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the protons in the following molecule appear at the highest ?-value in the 1H NMR spectrum? O
||
i ii iii iv
A) i
B) ii
C) iii
D) iv
||
i ii iii iv
A) i
B) ii
C) iii
D) iv

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
44
Which C9H10O compound gives the following 1H NMR spectrum? 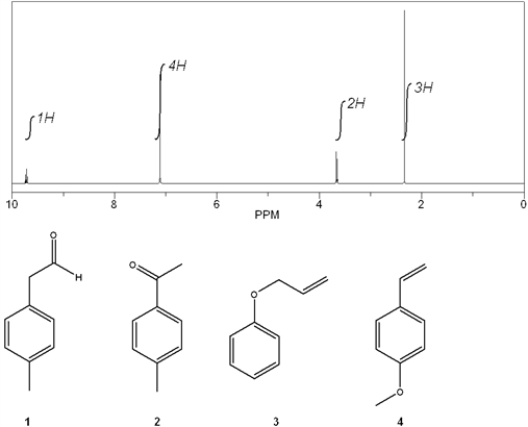
A) 1
B) 2
C) 3
D) 4

A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
45
Which C6H12O2 compound gives the following 1H NMR spectrum? 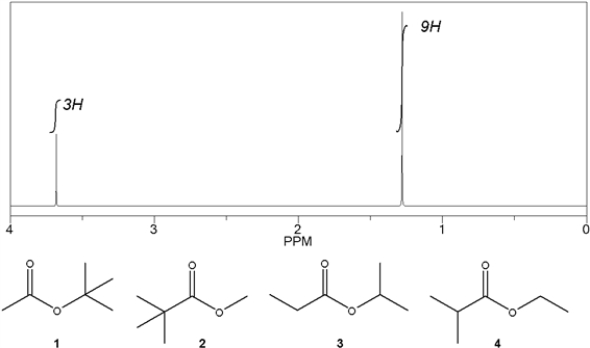
A) 1
B) 2
C) 3
D) 4

A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
46
Which C6H12O2 compound gives the following 1H NMR spectrum? 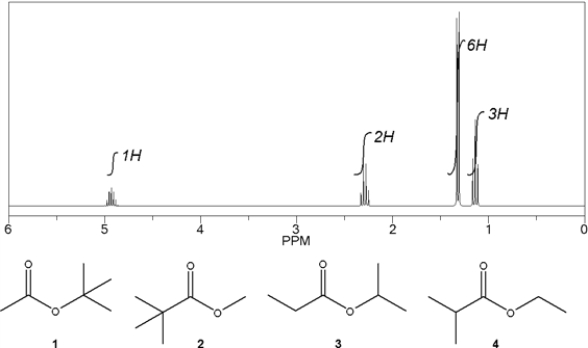
A) 1
B) 2
C) 3
D) 4

A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the carbon atoms in the following molecule appears furthest downfield in the 13C NMR spectrum? 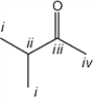
A) i
B) ii
C) iii
D) iv

A) i
B) ii
C) iii
D) iv

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
48
What is the hydrogen deficiency index for a compound with a molecular formula of C6H6Br2?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
49
What is the hydrogen deficiency index for a compound with a molecular formula of C12H16O?
A) 2
B) 4
C) 5
D) 6
A) 2
B) 4
C) 5
D) 6

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
50
The coupling constant, J, between the protons of chloroethane is 7 Hz when the spectrum is obtained at 250 MHz. What is the coupling constant between these protons when the spectrum is acquired at 500 MHz?
A) 3.5 Hz
B) 7 Hz
C) 14 Hz
D) 21 Hz
A) 3.5 Hz
B) 7 Hz
C) 14 Hz
D) 21 Hz

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following compounds gives a 1H NMR spectrum consisting of only a singlet, triplet and quintet?
A) CH3OCH2CH2CH2CH2OH
B) CH3OCH2CH2OCH2CH3
C) CH3OCH2CH2CH2OCH3
D) CH3OCH2CH(CH3)OCH3
A) CH3OCH2CH2CH2CH2OH
B) CH3OCH2CH2OCH2CH3
C) CH3OCH2CH2CH2OCH3
D) CH3OCH2CH(CH3)OCH3

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following compounds would give a 1H NMR spectrum consisting of two singlets and a 13C NMR consisting of consisting of three signals? 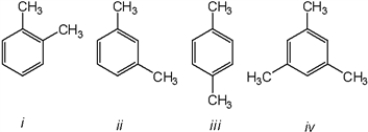
A) only i
B) only iii
C) only i and iii
D) iii and iv

A) only i
B) only iii
C) only i and iii
D) iii and iv

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
53
How many signals appear in the proton-decoupled 13C NMR spectrum of the following compound? 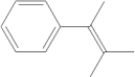
A) 7
B) 8
C) 9
D) 11

A) 7
B) 8
C) 9
D) 11

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following compounds gives a 1H NMR spectrum consisting of only two singlets?
A) CH3OCH2CH2OCH2CH3
B) CH3OCH2CH2CH2CH2OH
C) CH3OC(CH3)2OCH3
D) CH3OCH2CH(CH3)OCH3
A) CH3OCH2CH2OCH2CH3
B) CH3OCH2CH2CH2CH2OH
C) CH3OC(CH3)2OCH3
D) CH3OCH2CH(CH3)OCH3

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
55
What is the hydrogen deficiency index for a compound with a molecular formula of C10H16O2?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following compounds gives a 1H NMR spectrum consisting of only two triplets and a singlet?
A) CH3CH(OCH3)2
B) CH3OCH2CH2CH2CH2OCH3
C) CH3OCH2CH2OCH3
D) CH3OCH2CH(OH)CH3
A) CH3CH(OCH3)2
B) CH3OCH2CH2CH2CH2OCH3
C) CH3OCH2CH2OCH3
D) CH3OCH2CH(OH)CH3

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the carbon atoms in the molecule appears furthest downfield in the 13C NMR spectrum? 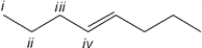
A) i
B) ii
C) iii
D) iv

A) i
B) ii
C) iii
D) iv

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
58
The chemical shift of the protons of acetone, CH3COCH3, is 2.1 ppm when the spectrum is obtained at 250 MHz. What is the chemical shift of these protons when the spectrum is acquired at 500 MHz?
A) 1.05 ppm
B) 2.1 ppm
C) 4.2 ppm
D) 8.4 ppm
A) 1.05 ppm
B) 2.1 ppm
C) 4.2 ppm
D) 8.4 ppm

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following compounds gives a 1H NMR spectrum consisting of only a singlet?
A) 1,1-dibromopropane
B) 1,2-dibromopropane
C) 1,3-dibromopropane
D) 2,2-dibromopropane
A) 1,1-dibromopropane
B) 1,2-dibromopropane
C) 1,3-dibromopropane
D) 2,2-dibromopropane

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
60
How many signals appear in the proton-decoupled 13C NMR spectrum of the following compound? 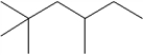
A) 6
B) 7
C) 8
D) 11

A) 6
B) 7
C) 8
D) 11

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
61
All of the following compound produce only singlets in a 1H NMR spectrum. 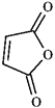
|
|


|
|

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
62
Predict the splitting of each of the numbered hydrogen atoms in the structure below in a 1H NMR spectrum. Identify as singlet, doublet, triplet, quartet, quintet, sextet, septet, .... Place the correct splitting in the blank to the left of the number. 
______ 1

______ 1

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
63
Which C8H10 compound gives the following 1H NMR spectrum? 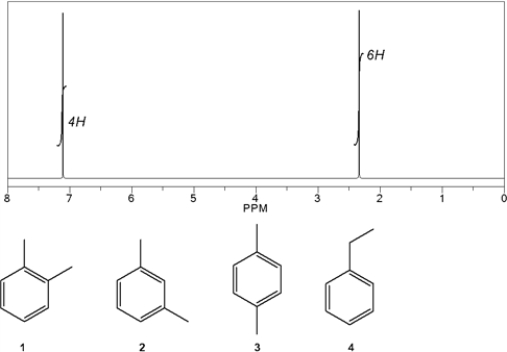
A) 1
B) 2
C) 3
D) 4

A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
64
Which C8H10 compound gives the following 1H NMR spectrum? 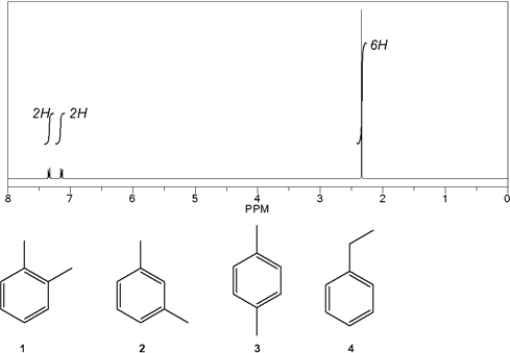
A) 1
B) 2
C) 3
D) 4

A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
65
For each of the compounds below tell how many signals you would expect the molecule to have in its normal, broadband hydrogen-decoupled 13C NMR spectra. Enter the numerical value in the blank provided to the left of the structure.
_______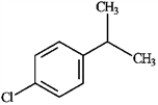
_______


Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
66
Nuclear magnetic resonance spectroscopy provides information about a molecule's carbon-hydrogen framework.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
67
For each of the compounds below tell how many signals you would expect the molecule to have in its normal, broadband hydrogen-decoupled 13C NMR spectra. Enter the numerical value in the blank provided to the left of the structure.
_______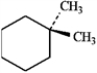
_______


Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
68
Consider the compound shown below. 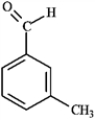 The above compound would produce a signal further downfield in a 13C NMR spectrum than
The above compound would produce a signal further downfield in a 13C NMR spectrum than 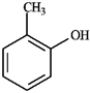
 The above compound would produce a signal further downfield in a 13C NMR spectrum than
The above compound would produce a signal further downfield in a 13C NMR spectrum than 

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
69
For each of the compounds below tell how many signals you would expect the molecule to have in its normal, broadband hydrogen-decoupled 13C NMR spectra. Enter the numerical value in the blank provided to the left of the structure.
_______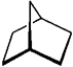
_______


Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
70
For each of the compounds below tell how many signals you would expect the molecule to have in its normal, broadband hydrogen-decoupled 13C NMR spectra. Enter the numerical value in the blank provided to the left of the structure.
_______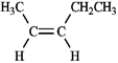
_______


Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
71
Consider the following structure. Answer the following questions by placing the appropriate number in the blank to the left. 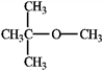
_____The number of nonequivalent hydrogen atoms in this compound.

_____The number of nonequivalent hydrogen atoms in this compound.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
72
Predict the splitting of each of the numbered hydrogen atoms in the structure below in a 1H NMR spectrum. Identify as singlet, doublet, triplet, quartet, quintet, sextet, septet, .... Place the correct splitting in the blank to the left of the number. 
______ 1

______ 1

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
73
Identify the ratio of peak areas upon integration of the 1H NMR spectrum for A, B, and C respectively. Enter the numbers separated by colons. 


Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
74
Consider the following structure. Answer the following questions by placing the appropriate number in the blank to the left. 
_____The number of signals in the hydrogen-decoupled 13C NMR of this compound.

_____The number of signals in the hydrogen-decoupled 13C NMR of this compound.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
75
The following compound will have a 1H NMR spectrum that consists of: 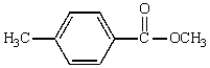 two triplets and a singlet, area ratio 3:3:4.
two triplets and a singlet, area ratio 3:3:4.
 two triplets and a singlet, area ratio 3:3:4.
two triplets and a singlet, area ratio 3:3:4.
Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
76
All of the following would produce nuclear magnetic resonance: 2H, 14N, 16O, and 19F.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
77
Predict the splitting of each of the numbered hydrogen atoms in the structure below in a 1H NMR spectrum. Identify as singlet, doublet, triplet, quartet, quintet, sextet, septet, .... Place the correct splitting in the blank to the left of the number. 
______ 1

______ 1

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
78
For each of the compounds below tell how many signals you would expect the molecule to have in its normal, broadband hydrogen-decoupled 13C NMR spectra. Enter the numerical value in the blank provided to the left of the structure.
_______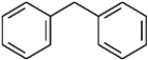
_______


Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
79
Consider the following structure. 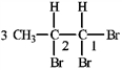 If the proton attached to C2 in 1,1,2-tribromopropane is coupled with the protons on C1 (J = 3.6 Hz) and C3 (J = 6.8), the tree diagram of the C2 proton is shown below.
If the proton attached to C2 in 1,1,2-tribromopropane is coupled with the protons on C1 (J = 3.6 Hz) and C3 (J = 6.8), the tree diagram of the C2 proton is shown below. 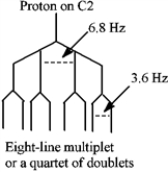
 If the proton attached to C2 in 1,1,2-tribromopropane is coupled with the protons on C1 (J = 3.6 Hz) and C3 (J = 6.8), the tree diagram of the C2 proton is shown below.
If the proton attached to C2 in 1,1,2-tribromopropane is coupled with the protons on C1 (J = 3.6 Hz) and C3 (J = 6.8), the tree diagram of the C2 proton is shown below. 

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
80
The splitting of signals in the 1H NMR spectrum provides information about the number of neighboring protons of each proton in the compound.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck



