Deck 6: Chemical Reactions: Energy, Rates, and Equilibrium
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/87
Play
Full screen (f)
Deck 6: Chemical Reactions: Energy, Rates, and Equilibrium
1
Which of the following is a potential consequence of misjudging the caloric needs of a patient?
A) malnourishment
B) muscle fatigue
C) respiratory failure
D) coma
E) All of the above are consequences.
A) malnourishment
B) muscle fatigue
C) respiratory failure
D) coma
E) All of the above are consequences.
All of the above are consequences.
2
The energy difference between I and III in the diagram is called 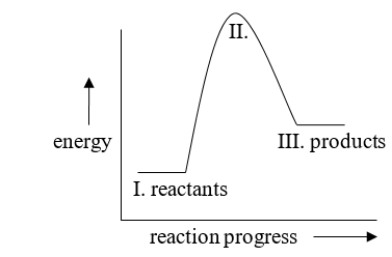
A) the activation energy
B) kinetic energy
C) heat of reaction
D) exothermic energy
E) endothermic energy

A) the activation energy
B) kinetic energy
C) heat of reaction
D) exothermic energy
E) endothermic energy
heat of reaction
3
How would this diagram be different if the reaction was exothermic? 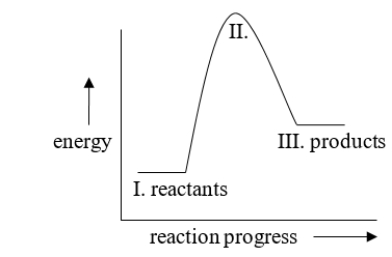
A) III would be lower than I
B) No difference; this reaction is already exothermic.
C) II would be lower than III.
D) II would be lower, between I and III.
E) II would be lower than I.

A) III would be lower than I
B) No difference; this reaction is already exothermic.
C) II would be lower than III.
D) II would be lower, between I and III.
E) II would be lower than I.
III would be lower than I
4
How is a biochemical pathway different than a biochemical reaction?
A) Actually, they are identical.
B) Only pathways occur in cells not reactions.
C) Only reactions occur in cells not pathways.
D) Reactions are catabolic, and pathways are anabolic.
E) A pathway is a specific sequence of reactions.
A) Actually, they are identical.
B) Only pathways occur in cells not reactions.
C) Only reactions occur in cells not pathways.
D) Reactions are catabolic, and pathways are anabolic.
E) A pathway is a specific sequence of reactions.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
5
The following reaction is a reversible reaction.Which statement BEST describes what it means for this reaction to be reversible? HCOOH + HOCH3 ⇌ HCOOCH3 + H2O
A) This reaction only occurs in the reverse direction as written above.
B) All of the reactant molecules react to make product, and then all of the product molecules react to make reactants again.
C) Forward and reverse reactions proceed at the same rate.
D) Forward and reverse reactions occur simultaneously.
E) The rate of the reverse reaction is must faster than the rate of the forward reaction.
A) This reaction only occurs in the reverse direction as written above.
B) All of the reactant molecules react to make product, and then all of the product molecules react to make reactants again.
C) Forward and reverse reactions proceed at the same rate.
D) Forward and reverse reactions occur simultaneously.
E) The rate of the reverse reaction is must faster than the rate of the forward reaction.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
6
A calorimeter is used to measure the energy content of foods because the energy measurement obtained using a calorimeter is the same as the energy we obtain by eating.Which of the following statements explains why this is the case?
A) They are the same because both the calorimeter and body combust food to obtain only heat energy.
B) They are the same because both the calorimeter and body combust food in a single exothermic step.
C) They are the same because both the calorimeter and body combust food in a complex series of steps.
D) They are the same because, overall, metabolism is a combustion reaction.
E) Actually, they are not the same values.
A) They are the same because both the calorimeter and body combust food to obtain only heat energy.
B) They are the same because both the calorimeter and body combust food in a single exothermic step.
C) They are the same because both the calorimeter and body combust food in a complex series of steps.
D) They are the same because, overall, metabolism is a combustion reaction.
E) Actually, they are not the same values.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
7
Combustion reactions are ___________ because products of the reaction are _________ in potential energy than the reactants.
A) endothermic; higher
B) exothermic; higher
C) endothermic; lower
D) exothermic; lower
E) endothermic; the same
A) endothermic; higher
B) exothermic; higher
C) endothermic; lower
D) exothermic; lower
E) endothermic; the same

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
8
During anabolism, heat is absorbed.What does the absorption of heat tell us about how these reactions occur?
A) These reactions do not usually occur because they require energy.
B) These reactions occur very quickly.
C) These reactions occur very slowly.
D) These reactions are driven by heat-releasing chemical reactions.
E) These reactions are not required by the body.
A) These reactions do not usually occur because they require energy.
B) These reactions occur very quickly.
C) These reactions occur very slowly.
D) These reactions are driven by heat-releasing chemical reactions.
E) These reactions are not required by the body.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following statements BEST describes catabolic pathways? I.Catabolic pathways build larger molecules from smaller ones.
II)Overall, catabolic pathways release energy.
III)An example of catabolism is when starch is broken down into smaller units of glucose during digestion.
A) I only
B) II only
C) III only
D) I and II
E) II and III
II)Overall, catabolic pathways release energy.
III)An example of catabolism is when starch is broken down into smaller units of glucose during digestion.
A) I only
B) II only
C) III only
D) I and II
E) II and III

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
10
Each of the following figures represents a reaction.Which reaction(s)has/have the slowest rate? 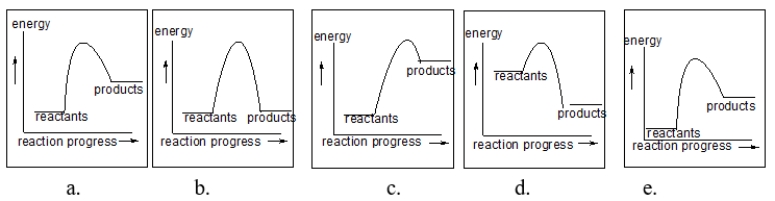
A) a and b
B) a, b, c, d and e
C) all but d
D) b only
E) b and e

A) a and b
B) a, b, c, d and e
C) all but d
D) b only
E) b and e

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following changes increases the rate of a reaction?
A) adding a catalyst
B) decreasing temperature
C) decreasing concentration
D) removing product as it is formed
E) All of the above increase the rate of a reaction.
A) adding a catalyst
B) decreasing temperature
C) decreasing concentration
D) removing product as it is formed
E) All of the above increase the rate of a reaction.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
12
How is the joule related to the calorie?
A) 1 cal = 4.184 J
B) 1 J = 4.184 cal
C) 1 Calorie = 4.184 J
D) 1 J = 4.184 Calorie
E) 1 J = 4.184 kcal
A) 1 cal = 4.184 J
B) 1 J = 4.184 cal
C) 1 Calorie = 4.184 J
D) 1 J = 4.184 Calorie
E) 1 J = 4.184 kcal

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
13
By moving, breathing, and living, you do work every day.Work requires energy.Where does this energy come from?
A) The energy comes from food; it undergoes chemical reactions in the cells.
B) The energy does not come from anyplace; it is always in our bodies.
C) The energy comes from breaking bonds in food.
D) The energy comes from breaking bonds in food, oxygen, and water.
E) No one really understands where this energy comes from.
A) The energy comes from food; it undergoes chemical reactions in the cells.
B) The energy does not come from anyplace; it is always in our bodies.
C) The energy comes from breaking bonds in food.
D) The energy comes from breaking bonds in food, oxygen, and water.
E) No one really understands where this energy comes from.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
14
One serving of animal crackers (30 g)contains 4.5 g of fat, 22 g of carbohydrates, and 2 g of proteins.Which of the following equations would you use to determine the number of calories in one serving of animal crackers?
A) 4.5 g × 4 Cal/g + 22 g × 9 Cal/g + 2 g × 4 Cal/g
B) 4.5 g × 9 Cal/g + 22 g × 4 Cal/g + 2 g × 4 Cal/g
C) 4.5 g × 4 Cal/g + 22 g × 4 Cal/g + 2 g × 4 Cal/g
D) 4.5 g × 9 Cal/g + 22 g × 9 Cal/g + 2 g × 4 Cal/g
E) 4.5 g × 4 Cal/g + 22 g × 4 Cal/g + 2 g × 9 Cal/g
A) 4.5 g × 4 Cal/g + 22 g × 9 Cal/g + 2 g × 4 Cal/g
B) 4.5 g × 9 Cal/g + 22 g × 4 Cal/g + 2 g × 4 Cal/g
C) 4.5 g × 4 Cal/g + 22 g × 4 Cal/g + 2 g × 4 Cal/g
D) 4.5 g × 9 Cal/g + 22 g × 9 Cal/g + 2 g × 4 Cal/g
E) 4.5 g × 4 Cal/g + 22 g × 4 Cal/g + 2 g × 9 Cal/g

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
15
An endothermic reaction is one that
A) releases heat.
B) has no heat of reaction.
C) absorbs heat.
D) has reactants higher in energy than products.
E) has a negative heat of reaction.
A) releases heat.
B) has no heat of reaction.
C) absorbs heat.
D) has reactants higher in energy than products.
E) has a negative heat of reaction.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
16
If a reaction has a ΔHrxn > 0, the reaction is
A) impossible.
B) exothermic.
C) endothermic.
D) explosive.
E) important in metabolism.
A) impossible.
B) exothermic.
C) endothermic.
D) explosive.
E) important in metabolism.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
17
A reaction occurs between the large black spheres and the small grey spheres.Each of the boxes below represents different reaction conditions for that reaction.Which box contains the reaction conditions that leads to the fastest reaction? 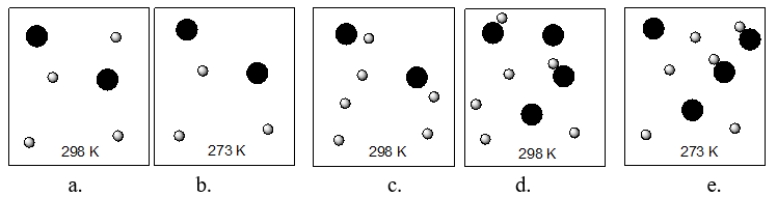
A) diagram a
B) diagram b
C) diagram c
D) diagram d
E) diagram e

A) diagram a
B) diagram b
C) diagram c
D) diagram d
E) diagram e

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
18
The sum of bond energies of bonds broken minus the sum of bond energies of bonds formed is the definition of
A) nutritional energy.
B) heat of reaction.
C) endothermic.
D) exothermic.
E) energy diagram.
A) nutritional energy.
B) heat of reaction.
C) endothermic.
D) exothermic.
E) energy diagram.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
19
Identify the false statement regarding bond energy.
A) Bonds with higher bond energies are stronger.
B) Stronger bonds have lower potential energy.
C) A single bond is stronger than a double bond.
D) A triple bond is stronger than a double bond.
E) Compounds with weaker bonds are more reactive.
A) Bonds with higher bond energies are stronger.
B) Stronger bonds have lower potential energy.
C) A single bond is stronger than a double bond.
D) A triple bond is stronger than a double bond.
E) Compounds with weaker bonds are more reactive.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
20
Combustion reactions are ___________ because products of the reaction are _________ in potential energy than the reactants.
A) endothermic; higher
B) exothermic; higher
C) endothermic; lower
D) exothermic; lower
E) endothermic; the same
A) endothermic; higher
B) exothermic; higher
C) endothermic; lower
D) exothermic; lower
E) endothermic; the same

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
21
An overview of metabolism is shown in the following figure.What does this figure tell us about the energy of catabolism and anabolism? 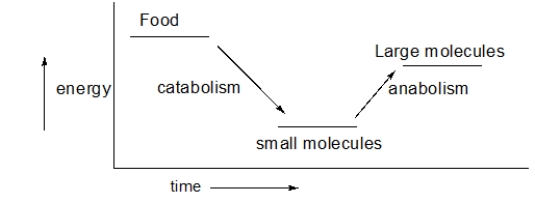
A) Anabolic reactions occur more quickly than catabolic reactions.
B) Catabolism is exothermic, and anabolism is endothermic.
C) Overall, we exert more energy than we consume.
D) Overall, the amount of energy that goes into anabolic processes is the same as is released in catabolic processes.
E) The conservation of energy does not apply to biological systems.

A) Anabolic reactions occur more quickly than catabolic reactions.
B) Catabolism is exothermic, and anabolism is endothermic.
C) Overall, we exert more energy than we consume.
D) Overall, the amount of energy that goes into anabolic processes is the same as is released in catabolic processes.
E) The conservation of energy does not apply to biological systems.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
22
What is a biochemical pathway?
A) the path that nutrients take through the body when they are metabolized
B) the complex blood transport system
C) a chemical reaction in the body that releases energy
D) a chemical reaction that releases carbon dioxide
E) a particular sequence of chemical reactions in the body
A) the path that nutrients take through the body when they are metabolized
B) the complex blood transport system
C) a chemical reaction in the body that releases energy
D) a chemical reaction that releases carbon dioxide
E) a particular sequence of chemical reactions in the body

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
23
What type of human calorimetry is the most practical, and why?
A) direct calorimetry because it is the most accurate
B) indirect calorimetry because it is the most accurate
C) direct calorimetry because it is portable and less expensive
D) indirect calorimetry because it is portable and less expensive
E) direct and indirect calorimetry are equally practical
A) direct calorimetry because it is the most accurate
B) indirect calorimetry because it is the most accurate
C) direct calorimetry because it is portable and less expensive
D) indirect calorimetry because it is portable and less expensive
E) direct and indirect calorimetry are equally practical

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
24
What are biological catalysts called?
A) proteins
B) nucleic acids
C) enzymes
D) carbohydrates
E) lipids
A) proteins
B) nucleic acids
C) enzymes
D) carbohydrates
E) lipids

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
25
The reaction of water with ammonia is shown below.What will happen if ammonium is added to the solution when it is at equilibrium? NH3 + H2O ⇌ NH4+ + OH-
ammonia water ammonium hydroxide
A) Nothing will happen.
B) The result is not predictable.
C) The ammonium will bubble.
D) The equilibrium will shift to the right.
E) The equilibrium will shift to the left.
ammonia water ammonium hydroxide
A) Nothing will happen.
B) The result is not predictable.
C) The ammonium will bubble.
D) The equilibrium will shift to the right.
E) The equilibrium will shift to the left.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
26
The ΔH for the reaction described by the energy diagram below is 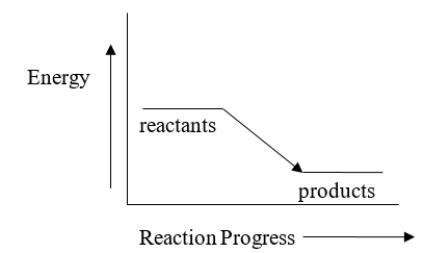
A) very large.
B) greater than zero but not necessarily very large.
C) exactly zero.
D) less than zero.
E) It is not possible to predict anything about the ΔH of this equation.

A) very large.
B) greater than zero but not necessarily very large.
C) exactly zero.
D) less than zero.
E) It is not possible to predict anything about the ΔH of this equation.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
27
A spirometer is used in indirect calorimetry.What does a spirometer measure?
A) oxygen
B) carbon dioxide
C) exhaled water
D) body heat
E) food intake
A) oxygen
B) carbon dioxide
C) exhaled water
D) body heat
E) food intake

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
28
An endothermic reaction absorbs heat when it occurs.How can the equilibrium of an endothermic reaction be shifted toward the products?
A) It is not possible to do this.
B) Add more products.
C) Cool down the reaction.
D) Add a catalyst.
E) Heat the reaction.
A) It is not possible to do this.
B) Add more products.
C) Cool down the reaction.
D) Add a catalyst.
E) Heat the reaction.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
29
During anabolism, heat is absorbed.What does the absorption of heat tell us about the relative energies of the starting small molecules and products of anabolism?
A) The products are lower energy than the reactants.
B) The reactants and products must be the same energy.
C) There is no consistent relationship between heat and the relative energies of the small molecules.
D) The reactants are lower energy than the products.
E) Actually, heat is not absorbed during anabolism.
A) The products are lower energy than the reactants.
B) The reactants and products must be the same energy.
C) There is no consistent relationship between heat and the relative energies of the small molecules.
D) The reactants are lower energy than the products.
E) Actually, heat is not absorbed during anabolism.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
30
The first law of thermodynamics states that
A) atoms are neither created nor destroyed during a chemical reaction.
B) energy is created when reactions release energy.
C) energy is conserved.
D) atoms are destroyed to produce energy from a chemical reaction.
E) kinetic energy is conserved but potential energy is not.
A) atoms are neither created nor destroyed during a chemical reaction.
B) energy is created when reactions release energy.
C) energy is conserved.
D) atoms are destroyed to produce energy from a chemical reaction.
E) kinetic energy is conserved but potential energy is not.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following processes are anabolic?
A) muscle building as a result of steroid use
B) releasing energy from food during the citric acid cycle
C) weight loss as a result of burning fat
D) transferring bond energy from food to ATP
E) All of the above are anabolic processes.
A) muscle building as a result of steroid use
B) releasing energy from food during the citric acid cycle
C) weight loss as a result of burning fat
D) transferring bond energy from food to ATP
E) All of the above are anabolic processes.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following statements describes an endothermic chemical reaction?
A) During photosynthesis, plants harvest light energy in order to drive the synthesis of carbohydrates from CO2 and H2O.
B) A natural gas burner can be used to heat water.
C) Melting an ice cube requires temperatures above freezing.
D) Metabolizing food supplies the body with the energy required to heat ourselves and move.
E) Exposing water to cold temperatures results in freezing.
A) During photosynthesis, plants harvest light energy in order to drive the synthesis of carbohydrates from CO2 and H2O.
B) A natural gas burner can be used to heat water.
C) Melting an ice cube requires temperatures above freezing.
D) Metabolizing food supplies the body with the energy required to heat ourselves and move.
E) Exposing water to cold temperatures results in freezing.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following reactions could be described by the energy diagram below? 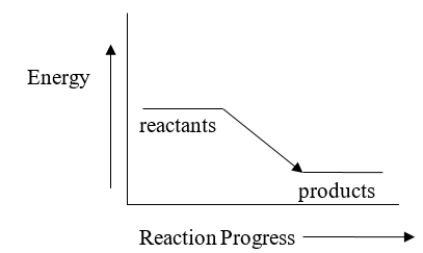
A) 2 CO2 + 556 kJ→ 2 CO + O2
B) CH4 + 2 O2 → CO2 + 2 H2O + heat
C) 8 H2S + heat → 8 H2 + S8
D) 6 CO2 + 6 H2O + heat → C6H12O6 + 6 O2
E) All of the reactions can be described by this energy diagram.

A) 2 CO2 + 556 kJ→ 2 CO + O2
B) CH4 + 2 O2 → CO2 + 2 H2O + heat
C) 8 H2S + heat → 8 H2 + S8
D) 6 CO2 + 6 H2O + heat → C6H12O6 + 6 O2
E) All of the reactions can be described by this energy diagram.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
34
During catabolism of food, heat is released.Where does this energy go?
A) It is turned into heat energy.
B) It is used in movement.
C) It is used to drive additional chemical reactions.
D) It is transferred to ATP.
E) All of the above are possible.
A) It is turned into heat energy.
B) It is used in movement.
C) It is used to drive additional chemical reactions.
D) It is transferred to ATP.
E) All of the above are possible.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
35
The chemical reaction in the diagram below is a(n)_____________ reaction. 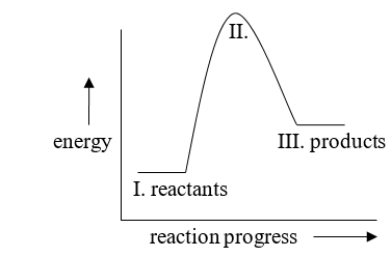
A) activated
B) kinetic
C) heated
D) exothermic
E) endothermic

A) activated
B) kinetic
C) heated
D) exothermic
E) endothermic

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following molecules is a special energy carrier molecule?
A) DNA
B) adenosine triphosphate
C) citric acid
D) insulin
E) starch
A) DNA
B) adenosine triphosphate
C) citric acid
D) insulin
E) starch

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following diagrams illustrates an endothermic reaction? 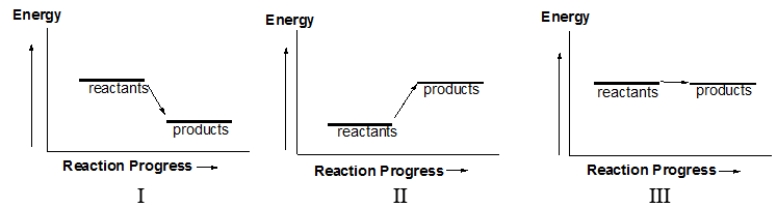
A) I and III
B) II and III
C) I and II
D) I only
E) II only

A) I and III
B) II and III
C) I and II
D) I only
E) II only

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
38
Which part of the following energy diagram is changed when a catalyst is added, and how is it changed? 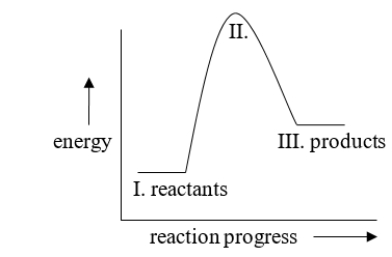
A) I is higher.
B) II is higher.
C) III is higher.
D) I is lower.
E) II is lower.

A) I is higher.
B) II is higher.
C) III is higher.
D) I is lower.
E) II is lower.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following biological molecules are the major nutrients that make up the food that we eat? I. Proteins
II.Nucleic acids
III.Steroids
IV.Fats
V.Carbohydrates
A) All of these are major nutrients.
B) I, II, IV, and V
C) I and V
D) III, IV, and V
E) I, IV, and V
II.Nucleic acids
III.Steroids
IV.Fats
V.Carbohydrates
A) All of these are major nutrients.
B) I, II, IV, and V
C) I and V
D) III, IV, and V
E) I, IV, and V

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following statements describe how oxygen intake relates to caloric requirements? I.Oxygen reacts with food to produce energy.
II)The larger the oxygen intake, the higher the energy output.
III)Oxygen is a product of food metabolism.
A) All of these statements describe the relationship between oxygen intake and caloric requirements.
B) I and II describe the relationship between oxygen intake and caloric requirements.
C) II and III describe the relationship between oxygen intake and caloric requirements.
D) I and III describe the relationship between oxygen intake and caloric requirements.
E) Only II describes the relationship between oxygen intake and caloric requirements.
II)The larger the oxygen intake, the higher the energy output.
III)Oxygen is a product of food metabolism.
A) All of these statements describe the relationship between oxygen intake and caloric requirements.
B) I and II describe the relationship between oxygen intake and caloric requirements.
C) II and III describe the relationship between oxygen intake and caloric requirements.
D) I and III describe the relationship between oxygen intake and caloric requirements.
E) Only II describes the relationship between oxygen intake and caloric requirements.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
41
Which statement describes reaction rate?
A) Reaction rate is how fast a reaction proceeds.
B) Reaction rate is measured by the disappearance of reactants over time.
C) Reaction rate is measured by the appearance of products formed over time.
D) Reaction rate is determined, in part, by activation energy.
E) All of the above statements describe reaction rate.
A) Reaction rate is how fast a reaction proceeds.
B) Reaction rate is measured by the disappearance of reactants over time.
C) Reaction rate is measured by the appearance of products formed over time.
D) Reaction rate is determined, in part, by activation energy.
E) All of the above statements describe reaction rate.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
42
Metabolism is
A) the breakdown of food.
B) the citric acid cycle.
C) the process by which fat is stored or burned.
D) all catabolic and anabolic pathways in the body.
E) the tendency to gain or lose weight.
A) the breakdown of food.
B) the citric acid cycle.
C) the process by which fat is stored or burned.
D) all catabolic and anabolic pathways in the body.
E) the tendency to gain or lose weight.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following reactions releases the smallest amount of heat? 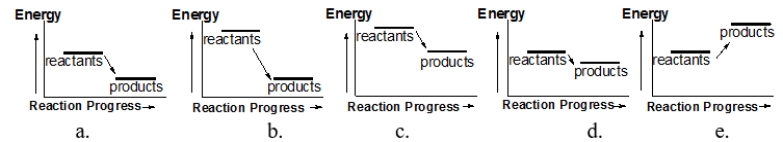
A) diagram a
B) diagram b
C) diagram c
D) diagram d
E) diagram e

A) diagram a
B) diagram b
C) diagram c
D) diagram d
E) diagram e

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
44
Carbohydrates and fats are sources of energy for cells because
A) they contain many C-C and C-H bonds, which are high in potential energy.
B) they are easy to metabolize.
C) they are readily absorbed from food.
D) they contain many C-C and C-H bonds, which are low in potential energy.
E) Both a and c are correct.
A) they contain many C-C and C-H bonds, which are high in potential energy.
B) they are easy to metabolize.
C) they are readily absorbed from food.
D) they contain many C-C and C-H bonds, which are low in potential energy.
E) Both a and c are correct.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
45
In order for a reaction to occur, reactant molecules must attain a certain amount of energy called _______.
A) ΔH
B) energy
C) entropy
D) activation energy
E) Any of the above.
A) ΔH
B) energy
C) entropy
D) activation energy
E) Any of the above.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
46
A hot dog contains 151 Calories.How many joules of energy is this?
A) 36 joules
B) 632 joules
C) 632,000 joules
D) 3.6 × 104 joules
E) 360 joules
A) 36 joules
B) 632 joules
C) 632,000 joules
D) 3.6 × 104 joules
E) 360 joules

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
47
The reaction, shown below, that occurs in a hydrogen fuel cell is exothermic.According to the conservation of energy, the reverse reaction (the electrolysis of water) 2 H2 + O2 → 2 H2O hydrogen fuel cell
2 H2O → 2 H2 + O2 electrolysis of water
A) is also exothermic.
B) is endothermic.
C) does not involve energy.
D) has an energy change of zero.
E) involves energy in some way, but it is impossible to determine how.
2 H2O → 2 H2 + O2 electrolysis of water
A) is also exothermic.
B) is endothermic.
C) does not involve energy.
D) has an energy change of zero.
E) involves energy in some way, but it is impossible to determine how.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
48
The nutritional unit of energy is the
A) calorie.
B) Calorie or kcal.
C) joule.
D) thermal unit.
E) heat of reaction.
A) calorie.
B) Calorie or kcal.
C) joule.
D) thermal unit.
E) heat of reaction.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
49
Which statement best describes the relationship between the heat of reaction and reaction rate?
A) There is no readily predictable relationship between ΔH and reaction rate.
B) Exothermic reactions are always slow.
C) Endothermic reactions are always slow.
D) The heat of reaction is always larger than reaction rate.
E) The relationship depends on whether the reaction is endothermic or exothermic.
A) There is no readily predictable relationship between ΔH and reaction rate.
B) Exothermic reactions are always slow.
C) Endothermic reactions are always slow.
D) The heat of reaction is always larger than reaction rate.
E) The relationship depends on whether the reaction is endothermic or exothermic.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following reactions releases the most heat? 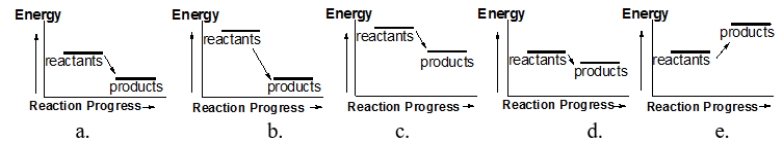
A) diagram a
B) diagram b
C) diagram c
D) diagram d
E) diagram e

A) diagram a
B) diagram b
C) diagram c
D) diagram d
E) diagram e

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
51
Increasing the concentration of the reactants causes the:
A) reactants to move faster.
B) reactants to collide more frequently.
C) reactants to collide with more energy.
D) rate of reaction to decrease.
E) All of the above result from increasing the concentration.
A) reactants to move faster.
B) reactants to collide more frequently.
C) reactants to collide with more energy.
D) rate of reaction to decrease.
E) All of the above result from increasing the concentration.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
52
Compounds that are used as fuels
A) contain C-C bonds.
B) contain C-H bonds.
C) are high in potential energy.
D) contain weak bonds.
E) All of these are used as fuels.
A) contain C-C bonds.
B) contain C-H bonds.
C) are high in potential energy.
D) contain weak bonds.
E) All of these are used as fuels.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
53
One serving (45 g)of dry wild rice contains 1.5 g of fat, 35 g of carbohydrates, and 5 g of protein.How many Calories does a serving of wild rice contain?
A) 166 Calories
B) 170 Calories
C) 200 Calories
D) 340 Calories
E) 370 Calories
A) 166 Calories
B) 170 Calories
C) 200 Calories
D) 340 Calories
E) 370 Calories

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
54
Each of the following figures represents a reaction.Which reaction has the fastest rate? 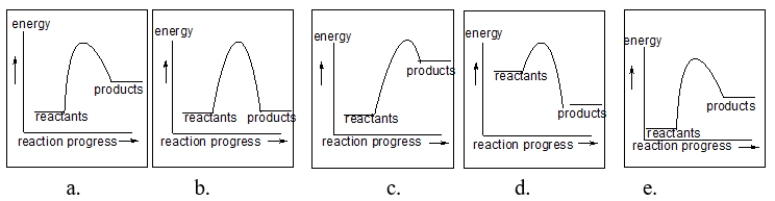
A) diagram a
B) diagram b
C) diagram c
D) diagram d
E) diagram e

A) diagram a
B) diagram b
C) diagram c
D) diagram d
E) diagram e

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
55
Why does increasing the concentration of reactants typically increase the rate of a reaction?
A) A higher concentration of reactants means a higher kinetic energy per molecule.
B) A higher concentration of reactants means a lower activation energy.
C) A higher concentration of reactants means that a catalyst has been added.
D) A higher concentration of reactants means a higher activation energy.
E) A higher concentration of reactants means more collisions per amount of time.
A) A higher concentration of reactants means a higher kinetic energy per molecule.
B) A higher concentration of reactants means a lower activation energy.
C) A higher concentration of reactants means that a catalyst has been added.
D) A higher concentration of reactants means a higher activation energy.
E) A higher concentration of reactants means more collisions per amount of time.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following reactions absorbs heat? 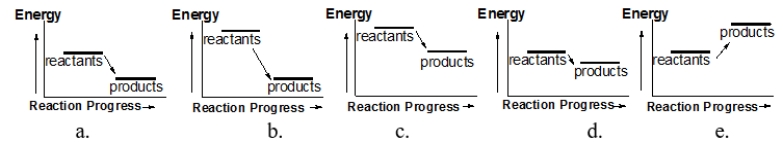
A) diagram a
B) diagram b
C) diagram c
D) diagram d
E) diagram e

A) diagram a
B) diagram b
C) diagram c
D) diagram d
E) diagram e

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
57
The name for the heat energy released or absorbed in a chemical reaction is
A) energy.
B) endothermic.
C) exothermic.
D) heat of reaction.
E) entropy.
A) energy.
B) endothermic.
C) exothermic.
D) heat of reaction.
E) entropy.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
58
When bonds break, energy is
A) always released.
B) always absorbed.
C) typically released but sometimes absorbed.
D) typically absorbed but sometimes released.
E) absorbed half the time and released half the time.
A) always released.
B) always absorbed.
C) typically released but sometimes absorbed.
D) typically absorbed but sometimes released.
E) absorbed half the time and released half the time.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
59
How does increasing the temperature of a reaction affect the reaction rate?
A) It slows the rate down by changing the heat of reaction.
B) It speeds the rate up by raising the activation energy.
C) It slows the rate down by increasing the activation energy.
D) It speeds the rate up by increasing the kinetic energy of the molecules.
E) It slows the rate down by increasing the kinetic energy of the molecules.
A) It slows the rate down by changing the heat of reaction.
B) It speeds the rate up by raising the activation energy.
C) It slows the rate down by increasing the activation energy.
D) It speeds the rate up by increasing the kinetic energy of the molecules.
E) It slows the rate down by increasing the kinetic energy of the molecules.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
60
How many nutritional Calories are in a food sample containing 550 kcal?
A) 5,500,000
B) 550,000
C) 5,500
D) 550
E) 55
A) 5,500,000
B) 550,000
C) 5,500
D) 550
E) 55

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
61
Select the TRUE statement concerning rate of reactions.
A) A large activation energy indicates high heat of reaction.
B) A small activation energy indicates high heat of reaction.
C) A large activation energy indicates low heat of reaction.
D) A small activation energy indicates low heat of reaction.
E) The activation energy is independent of the heat of reaction.
A) A large activation energy indicates high heat of reaction.
B) A small activation energy indicates high heat of reaction.
C) A large activation energy indicates low heat of reaction.
D) A small activation energy indicates low heat of reaction.
E) The activation energy is independent of the heat of reaction.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
62
Which statement BEST describes how calorimetry is used in medicine?
A) Calorimetry is used to feed patients.
B) Calorimetry is used to measure the temperature of a patient.
C) Calorimetry is used to measure the oxygen needs of a patient.
D) Calorimetry is used to assess the caloric needs of a patient.
E) All of the above describe calorimetry use in medicine.
A) Calorimetry is used to feed patients.
B) Calorimetry is used to measure the temperature of a patient.
C) Calorimetry is used to measure the oxygen needs of a patient.
D) Calorimetry is used to assess the caloric needs of a patient.
E) All of the above describe calorimetry use in medicine.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
63
Which process that occurs during this reaction gives off heat? 2 H2 + O2 → 2 H2O
A) breaking H-H
B) breaking O-O
C) breaking O-H
D) forming O-H
E) both breaking and forming bonds
A) breaking H-H
B) breaking O-O
C) breaking O-H
D) forming O-H
E) both breaking and forming bonds

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
64
Select the correct statement regarding the reactants and products in the reaction represented by the energy diagram below. 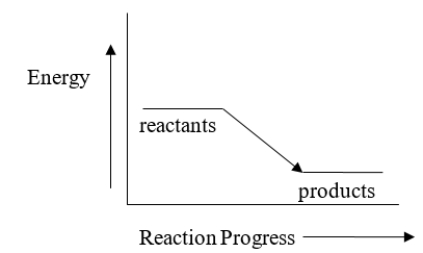
A) The products have more energy than the reactants.
B) The reactants are more stable than the products.
C) The products are more stable than the reactants.
D) The reactants and products have the same energy.
E) The products are less stable than the reactants.

A) The products have more energy than the reactants.
B) The reactants are more stable than the products.
C) The products are more stable than the reactants.
D) The reactants and products have the same energy.
E) The products are less stable than the reactants.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following diagrams illustrates an exothermic reaction? 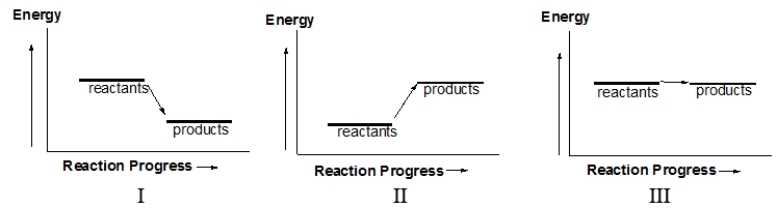
A) I and III
B) II and III
C) I and II
D) I only
E) II only

A) I and III
B) II and III
C) I and II
D) I only
E) II only

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
66
Atwater and Rosa built the first human calorimeter in the 1890s.After measuring the energy expenditure and food and oxygen intake of humans, they made a key discovery about the transformation of matter and energy in the human body.What discovery did they make?
A) Amazingly, humans exert more energy than they take in.
B) Humans are inefficient and don't exert as much energy as they take in.
C) They confirmed that the conservation of energy applies to humans.
D) Direct calorimetry is the gold standard for accurate measurement.
E) All of the above were discovered.
A) Amazingly, humans exert more energy than they take in.
B) Humans are inefficient and don't exert as much energy as they take in.
C) They confirmed that the conservation of energy applies to humans.
D) Direct calorimetry is the gold standard for accurate measurement.
E) All of the above were discovered.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
67
Which statement BEST describes how the temperature of the surroundings changes as a result of the reaction represented by energy diagram below? 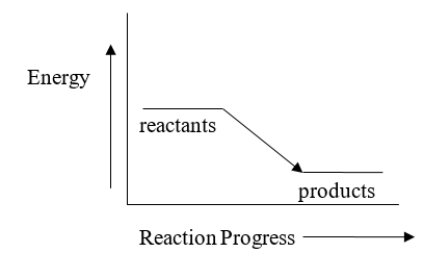
A) The temperature of the surroundings does not change.
B) The temperature of the surroundings increases.
C) The temperature of the surroundings decreases.
D) The surroundings will become very cold.
E) It is not possible to predict anything about the temperature of the surroundings based on this diagram.

A) The temperature of the surroundings does not change.
B) The temperature of the surroundings increases.
C) The temperature of the surroundings decreases.
D) The surroundings will become very cold.
E) It is not possible to predict anything about the temperature of the surroundings based on this diagram.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
68
Select the TRUE statement concerning rate of reactions.
A) A large activation energy indicates a fast reaction.
B) A catalyst increases the activation energy.
C) Low temperatures help reactants to collide with each other.
D) Slow reactions have high activation energies.
E) All of the above statements are true.
A) A large activation energy indicates a fast reaction.
B) A catalyst increases the activation energy.
C) Low temperatures help reactants to collide with each other.
D) Slow reactions have high activation energies.
E) All of the above statements are true.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
69
Bond breaking is always endothermic and yet catabolism, the breakdown of nutrients, is an overall exothermic process.How can you reconcile these two statements?
A) Catabolism is an exception to the rule.
B) Bond breaking is not really always endothermic.
C) Endothermic bond breaking is only seen in the laboratory.
D) No one really understands this.
E) Catabolism is the sum of many reactions, including many bond-making reactions.
A) Catabolism is an exception to the rule.
B) Bond breaking is not really always endothermic.
C) Endothermic bond breaking is only seen in the laboratory.
D) No one really understands this.
E) Catabolism is the sum of many reactions, including many bond-making reactions.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
70
The reaction between acetic acid (CH3COOH)and methanol (CH3OH)is given below, followed by a list of changes that could be made to the reaction.Which of these changes will result in the equilibrium shifting to the left? CH3COOH + CH3OH ⇌ CH3COOCH3 + H2O
Changes that could be made to the solution
I.Adding more CH3COOH
II.Removing H2O
III.Adding more CH3COOCH3
IV.Removing CH3OH
A) All of these changes will result in the equilibrium shifting to the left.
B) Only I will result in the equilibrium shifting to the left.
C) Only IV will result in the equilibrium shifting to the left.
D) I and III will result in the equilibrium shifting to the left.
E) III and IV will result in the equilibrium shifting to the left.
Changes that could be made to the solution
I.Adding more CH3COOH
II.Removing H2O
III.Adding more CH3COOCH3
IV.Removing CH3OH
A) All of these changes will result in the equilibrium shifting to the left.
B) Only I will result in the equilibrium shifting to the left.
C) Only IV will result in the equilibrium shifting to the left.
D) I and III will result in the equilibrium shifting to the left.
E) III and IV will result in the equilibrium shifting to the left.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
71
Increasing the temperature of an exothermic reaction at equilibrium results in
A) a shift to the right.
B) a shift to the left.
C) products being produced more quickly.
D) reactants being used up more quickly.
E) Both c and d are correct.
A) a shift to the right.
B) a shift to the left.
C) products being produced more quickly.
D) reactants being used up more quickly.
E) Both c and d are correct.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
72
Which statement is the BEST definition of chemical equilibrium?
A) the point at which the mass of reaction products equals the mass of the reactants
B) the point at which forward and reverse reactions proceed at the same rate
C) the point at which a reaction is balanced
D) the point at which the number of moles of product equals the number of moles of reactants
E) Both answers b and d correctly define equilibrium.
A) the point at which the mass of reaction products equals the mass of the reactants
B) the point at which forward and reverse reactions proceed at the same rate
C) the point at which a reaction is balanced
D) the point at which the number of moles of product equals the number of moles of reactants
E) Both answers b and d correctly define equilibrium.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
73
Select the TRUE statement concerning a reaction at equilibrium.
A) The forward reaction continues to convert reactants into products.
B) The reverse reaction continues to convert products into reactants.
C) The rate of the forward reaction is the same as the rate of the reverse reaction.
D) The concentration of each reactant and product is constant.
E) All of the above are true concerning a reaction at equilibrium.
A) The forward reaction continues to convert reactants into products.
B) The reverse reaction continues to convert products into reactants.
C) The rate of the forward reaction is the same as the rate of the reverse reaction.
D) The concentration of each reactant and product is constant.
E) All of the above are true concerning a reaction at equilibrium.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
74
The energy difference between I and II in the diagram is called 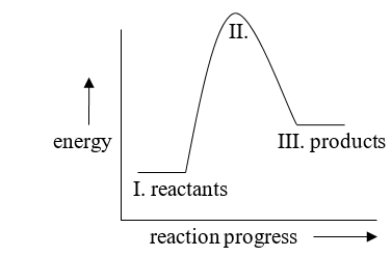
A) the activation energy
B) kinetic energy
C) heat of reaction
D) exothermic energy
E) endothermic energy

A) the activation energy
B) kinetic energy
C) heat of reaction
D) exothermic energy
E) endothermic energy

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
75
How is the reaction that occurs in a calorimeter different from human metabolism?
A) One is a combustion reaction, and the other is not.
B) One occurs in many steps, and the other does not.
C) One is exothermic, and the other is endothermic.
D) One releases carbon dioxide, and the other does not.
E) These reactions are exactly the same.
A) One is a combustion reaction, and the other is not.
B) One occurs in many steps, and the other does not.
C) One is exothermic, and the other is endothermic.
D) One releases carbon dioxide, and the other does not.
E) These reactions are exactly the same.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
76
Which bonds are broken, and which bonds are formed during this chemical reaction? 2 H2 + O2 → 2 H2O
A) Broken: H-H and O-O Formed: O-H
B) Broken: H-H Formed: O-H and O-O
C) Broken: O-O Formed: O-H and H-H
D) Broken: O-H Formed: O-O and H-H
E) Broken: O-H Formed: O-H
A) Broken: H-H and O-O Formed: O-H
B) Broken: H-H Formed: O-H and O-O
C) Broken: O-O Formed: O-H and H-H
D) Broken: O-H Formed: O-O and H-H
E) Broken: O-H Formed: O-H

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
77
How many joules of energy are contained in 350 cal?
A) 350 J
B) 1,500 J
C) 1,466.2 J
D) 84 J
E) 83.65 J
A) 350 J
B) 1,500 J
C) 1,466.2 J
D) 84 J
E) 83.65 J

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
78
Increasing the temperature of a reaction causes the
A) reactants to move faster.
B) reactants to collide more frequently.
C) reactants to collide with more energy.
D) rate of reaction to increase.
E) All of the above result from increasing the temperature.
A) reactants to move faster.
B) reactants to collide more frequently.
C) reactants to collide with more energy.
D) rate of reaction to increase.
E) All of the above result from increasing the temperature.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
79
Convert 3.5 × 104 J to calories.
A) 8,365 calories
B) 0.84 calories
C) 8.4 × 103 calories
D) 1.5 × 105 calories
E) 14,644 calories
A) 8,365 calories
B) 0.84 calories
C) 8.4 × 103 calories
D) 1.5 × 105 calories
E) 14,644 calories

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
80
An exothermic reaction is one that:
A) has a positive ΔH.
B) has no ΔH.
C) absorbs heat.
D) has products higher in energy than reactants.
E) has a negative ΔH.
A) has a positive ΔH.
B) has no ΔH.
C) absorbs heat.
D) has products higher in energy than reactants.
E) has a negative ΔH.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck



