Deck 13: Heterocyclic Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/36
Play
Full screen (f)
Deck 13: Heterocyclic Compounds
1
Which of the following statements about pyridine is false?
A) The nitrogen is basic.
B) Pyridine reacts faster than benzene in electrophilic aromatic substitution reactions.
C) Pyridine can behave as a hydrogen bond acceptor.
D) Pyridine is more polar than benzene.
E) All of pyridine's atoms are sp2 hybridized.
A) The nitrogen is basic.
B) Pyridine reacts faster than benzene in electrophilic aromatic substitution reactions.
C) Pyridine can behave as a hydrogen bond acceptor.
D) Pyridine is more polar than benzene.
E) All of pyridine's atoms are sp2 hybridized.
Pyridine reacts faster than benzene in electrophilic aromatic substitution reactions.
2
Pyrrole is a weak base because: 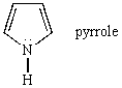
A) the nitrogen lone pair is part of the aromatic system.
B) the nitrogen lone pair is in an sp2-hybridized orbital.
C) the nitrogen lone pair is on a highly electronegative atom.
D) the nitrogen lone pair is easily deprotonated
E) the nitrogen lone pair is easily protonated.

A) the nitrogen lone pair is part of the aromatic system.
B) the nitrogen lone pair is in an sp2-hybridized orbital.
C) the nitrogen lone pair is on a highly electronegative atom.
D) the nitrogen lone pair is easily deprotonated
E) the nitrogen lone pair is easily protonated.
the nitrogen lone pair is part of the aromatic system.
3
The structural formula for furan is:
A)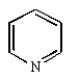
B)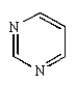
C)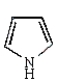
D)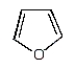
E)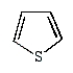
A)

B)

C)

D)

E)


4
The decreasing order of basicity of the following is: 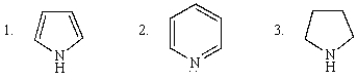
A) 1 > 2 > 3
B) 1 > 3 > 2
C) 3 > 2 > 1
D) 2 > 1 > 3
E) 3 > 1 > 2

A) 1 > 2 > 3
B) 1 > 3 > 2
C) 3 > 2 > 1
D) 2 > 1 > 3
E) 3 > 1 > 2

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
5
What type of heterocycle is present in both histamine and histidine? 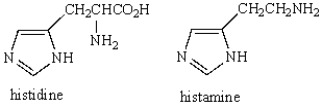
A) indole
B) pyrimidine
C) purine
D) imidazole
E) thiazole

A) indole
B) pyrimidine
C) purine
D) imidazole
E) thiazole

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
6
The name of the heterocycle below is: 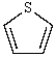
A) pyrrole
B) furan
C) thiophene
D) pyryllium
E) phenol

A) pyrrole
B) furan
C) thiophene
D) pyryllium
E) phenol

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following molecules can be classified as a heterocycle?
A)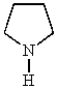
B)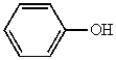
C)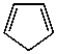
D)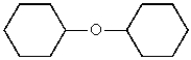
E)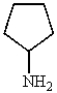
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is not a heterocyclic compound? 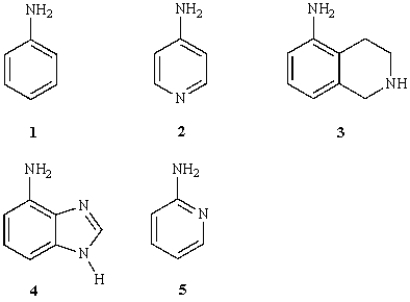
A) 1
B) 2
C) 3
D) 4
E) 5

A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following statements about the pyrylium ion is false? 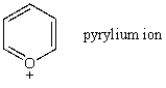
A) The pyrylium ion appears as a substructure in many pigments.
B) The pyrylium ion is an example of an aromatic cation.
C) The oxygen lone pair in a pyrylium ion is basic.
D) All atoms in the pyrylium ion (except the hydrogens) are sp2-hybridized.
E) The pyrylium ion will react with nucleophiles.

A) The pyrylium ion appears as a substructure in many pigments.
B) The pyrylium ion is an example of an aromatic cation.
C) The oxygen lone pair in a pyrylium ion is basic.
D) All atoms in the pyrylium ion (except the hydrogens) are sp2-hybridized.
E) The pyrylium ion will react with nucleophiles.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
10
What is the structure of 2,5-dimethylpyrrole?
A)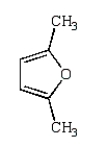
B)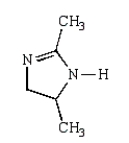
C)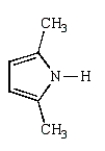
D)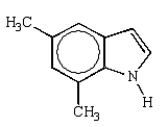
E)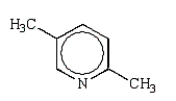
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
11
The name of the heterocycle below is: 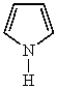
A) pyrrole
B) furan
C) thiophene
D) pyryllium
E) phenol

A) pyrrole
B) furan
C) thiophene
D) pyryllium
E) phenol

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
12
What type of heterocycle is found in guanine? 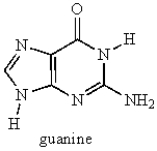
A) purine
B) pyrimidine
C) pyridine
D) quinoline
E) oxazole

A) purine
B) pyrimidine
C) pyridine
D) quinoline
E) oxazole

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
13
In imidazole, 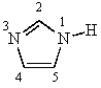
A) the chemistry is more like a diene than like an aromatic compound.
B) the chemistry is more like an isolated alkene than an aromatic compound.
C) N1 is more basic than N3.
D) both N3 and N1 are equally basic.
E) N3 is more basic than N1.

A) the chemistry is more like a diene than like an aromatic compound.
B) the chemistry is more like an isolated alkene than an aromatic compound.
C) N1 is more basic than N3.
D) both N3 and N1 are equally basic.
E) N3 is more basic than N1.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
14
The heterocycle cytosine, found in nucleic acids, is an example of a:
A) purine
B) pyrimidine
C) pyridine
D) piperidine
E) pyrazine
A) purine
B) pyrimidine
C) pyridine
D) piperidine
E) pyrazine

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
15
To what family of heterocyclic compounds do both serotonin and tryptophan belong? 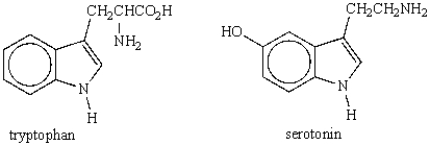
A) imidazole
B) oxazole
C) thiazole
D) indole
E) pyridazine

A) imidazole
B) oxazole
C) thiazole
D) indole
E) pyridazine

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
16
The structure of quinoline is:
A)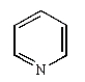
B)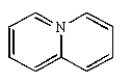
C)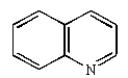
D)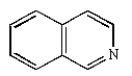
E)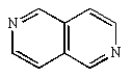
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following statements is false?
A) Furan, pyrrole, and thiophene are more reactive than benzene in electrophilic aromatic substitution.
B) Electrophilic substitution in pyridine occurs mainly in the 2- and 4-positions.
C) Electrophilic substitution in furan, pyrrole, and thiophene occurs mainly in the 2-position.
D) Pyridine is more basic than pyrrole.
E) An unshared electron pair on O, N, or S is part of the aromatic system in furan, pyrrole, and thiophene.
A) Furan, pyrrole, and thiophene are more reactive than benzene in electrophilic aromatic substitution.
B) Electrophilic substitution in pyridine occurs mainly in the 2- and 4-positions.
C) Electrophilic substitution in furan, pyrrole, and thiophene occurs mainly in the 2-position.
D) Pyridine is more basic than pyrrole.
E) An unshared electron pair on O, N, or S is part of the aromatic system in furan, pyrrole, and thiophene.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
18
Pyridine is a weaker base than aliphatic tertiary amines because
A) the N is sp2 hybridized in pyridine and sp3 hybridized in aliphatic amines.
B) there are no unshared electrons on the N in pyridine.
C) the electrons on the N in pyridine are delocalized around the ring.
D) the N in pyridine is already protonated.
E) the unshared electron pair on N in pyridine is part of the aromatic system.
A) the N is sp2 hybridized in pyridine and sp3 hybridized in aliphatic amines.
B) there are no unshared electrons on the N in pyridine.
C) the electrons on the N in pyridine are delocalized around the ring.
D) the N in pyridine is already protonated.
E) the unshared electron pair on N in pyridine is part of the aromatic system.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following heterocyclic compounds is not aromatic?
A)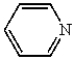
B)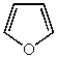
C)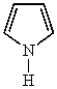
D)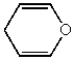
E)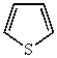
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
20
The name of 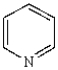 is:
is:
A) pyrrole
B) furan
C) pyridine
D) pyrimidine
E) thiophene
 is:
is:A) pyrrole
B) furan
C) pyridine
D) pyrimidine
E) thiophene

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
21
What is the major product of the following reaction? 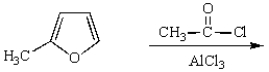
A)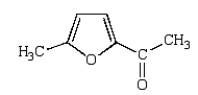
B)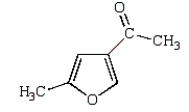
C)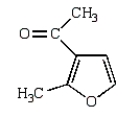
D)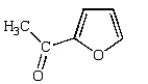
E)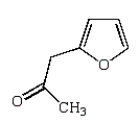
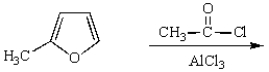
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following compounds will undergo nucleophilic aromatic substitution at the fastest rate?
A)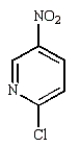
B)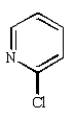
C)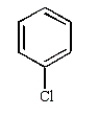
D)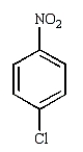
E)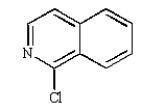
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
23
What reagent(s) will accomplish the following transformation? 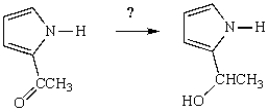
A) H2SO4 in H2O
B) NaOCH3 in CH3OH
C) NaBH4
D) CH3I
E) NaOH in H2O

A) H2SO4 in H2O
B) NaOCH3 in CH3OH
C) NaBH4
D) CH3I
E) NaOH in H2O

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
24
What set of reagents is needed to carry out the following reaction? 
A) KMnO4
B) 3 H2, Pt
C) NaNH2, liq.NH3
D) HCl, H2O
E) NaOH, H2O

A) KMnO4
B) 3 H2, Pt
C) NaNH2, liq.NH3
D) HCl, H2O
E) NaOH, H2O

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
25
The following reaction occurs by what mechanism? 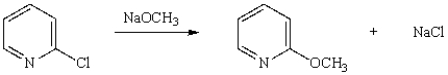
A) SN1
B) SN2
C) nucleophilic acyl substitution
D) nucleophilic aromatic substitution
E) electrophilic aromatic substitution

A) SN1
B) SN2
C) nucleophilic acyl substitution
D) nucleophilic aromatic substitution
E) electrophilic aromatic substitution

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following compounds will undergo electrophilic aromatic substitution reactions at the fastest rate?
A)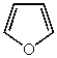
B)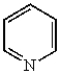
C)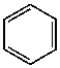
D)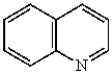
E)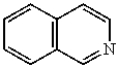
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
27
The product of the following reaction is 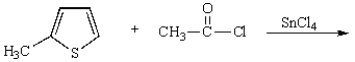
A)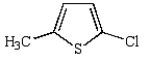
B)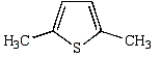
C)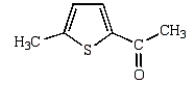
D)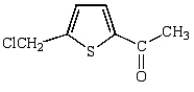
E)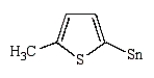
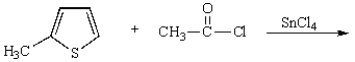
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
28
An intermediate in the nitration of pyridine is: 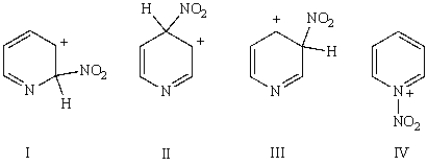
A) I
B) II
C) III
D) IV
E) none of the above

A) I
B) II
C) III
D) IV
E) none of the above

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
29
The first step in the following reaction is: 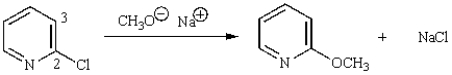
A) loss of chloride (Cl-) from C-2 to give a carbocation.
B) displacement of chloride (Cl-) from C-2 by methoxide (CH3O-).
C) base-promoted elimination of HCl from carbons 2 and 3.
D) reduction of the pyridine by methoxide.
E) addition of methoxide to C-2.

A) loss of chloride (Cl-) from C-2 to give a carbocation.
B) displacement of chloride (Cl-) from C-2 by methoxide (CH3O-).
C) base-promoted elimination of HCl from carbons 2 and 3.
D) reduction of the pyridine by methoxide.
E) addition of methoxide to C-2.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
30
What is the major product of the following reaction? 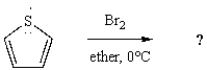
A)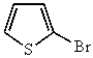
B)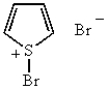
C)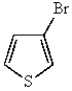
D)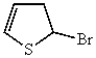
E)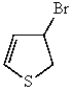
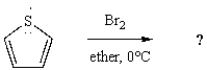
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
31
The final product of the following reaction sequence is: 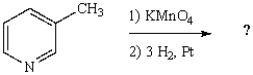
A)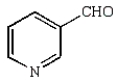
B)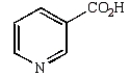
C)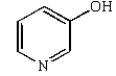
D)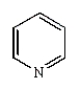
E)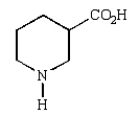

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
32
The product of the following reaction is: 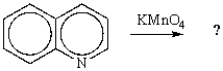
A)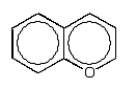
B)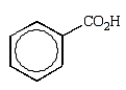
C)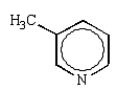
D)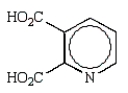
E)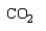

A)

B)

C)

D)

E)

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
33
What reagent is required to accomplish the following transformation? 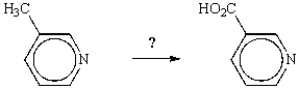
A) NaBH4
B) CH3OH
C) KMnO4
D) NaOCH3
E) NaNH2/NH3

A) NaBH4
B) CH3OH
C) KMnO4
D) NaOCH3
E) NaNH2/NH3

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
34
Furfural (2-furaldehyde) is produced by dehydrating a(n):
A) cyclic acid
B) ether
C) hexose
D) pentose
E) triose
A) cyclic acid
B) ether
C) hexose
D) pentose
E) triose

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
35
The product of the following reaction is 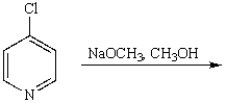
A)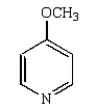
B)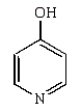
C)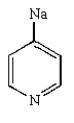
D)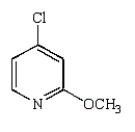
E)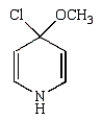
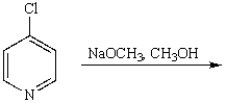
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
36
The major mechanism by which five membered aromatic heterocycles react with electrophiles is:
A) nucleophilic aromatic substitution
B) electrophilic aromatic substitution
C) ionic addition
D) free radical addition
E) oxidation-reduction
A) nucleophilic aromatic substitution
B) electrophilic aromatic substitution
C) ionic addition
D) free radical addition
E) oxidation-reduction

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck



