Deck 11: Amines and Related Nitrogen Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/40
Play
Full screen (f)
Deck 11: Amines and Related Nitrogen Compounds
1
Which compound is the strongest base?
A) CH3NH2
B) CH3CO2H
C) CH3CHO
D) CH3OH
E) C6H5NH2
A) CH3NH2
B) CH3CO2H
C) CH3CHO
D) CH3OH
E) C6H5NH2
CH3NH2
2
The mechanism by which acylation of an amine with an acid chloride takes place is:
A) nucleophilic acyl substitution
B) electrophilic aromatic substitution
C) nucleophilic addition
D) electrophilic addition
E) nucleophilic aromatic substitution
A) nucleophilic acyl substitution
B) electrophilic aromatic substitution
C) nucleophilic addition
D) electrophilic addition
E) nucleophilic aromatic substitution
nucleophilic acyl substitution
3
Which of the following compounds is a tertiary amine?
A)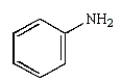
B)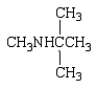
C)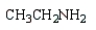
D)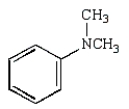
E)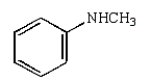
A)

B)

C)
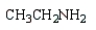
D)

E)


4
The structure below can be named in the following manner: 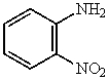
A) 2-nitrocyclohexanamine
B) m-nitroaniline
C) o-nitroaniline
D) 2-nitrobenzylamine
E) 1,2-diaminobenzene

A) 2-nitrocyclohexanamine
B) m-nitroaniline
C) o-nitroaniline
D) 2-nitrobenzylamine
E) 1,2-diaminobenzene

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following are tertiary amines? 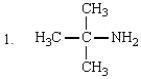
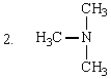

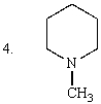
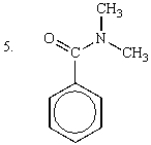
A) 1 and 3
B) only 5
C) 1, 2, 3, and 4
D) 2 and 4
E) 1 and 2





A) 1 and 3
B) only 5
C) 1, 2, 3, and 4
D) 2 and 4
E) 1 and 2

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following amines is the most basic?
A) methylamine
B) dimethylamine
C) ammonia
D) aniline
E) N-methylaniline
A) methylamine
B) dimethylamine
C) ammonia
D) aniline
E) N-methylaniline

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
7
What is the mechanism for the intramolecular alkylation shown below?
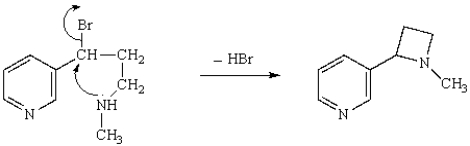
A) SN2
B) SN1
C) nucleophilic acyl substitution
D) nucleophilic addition
E) electrophilic addition

A) SN2
B) SN1
C) nucleophilic acyl substitution
D) nucleophilic addition
E) electrophilic addition

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
8
C6H5NHCH2CH2CH3 can best be prepared as follows:
A)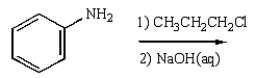
B)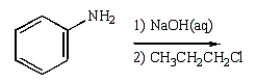
C)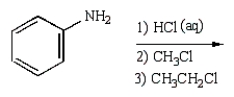
D)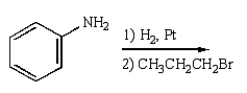
E)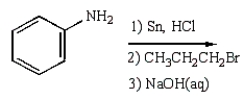
A)
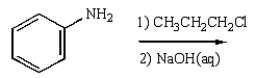
B)
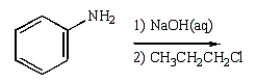
C)

D)
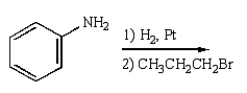
E)
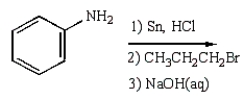

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following is not an intermediate in the diazotization of aniline using nitrous acid? 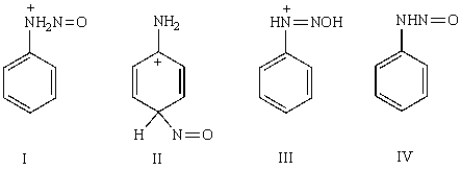
A) I
B) II
C) III
D) IV
E) none of the above

A) I
B) II
C) III
D) IV
E) none of the above

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
10
When methylamine (CH3NH2) reacts with methyl bromide (CH3Br) to give dimethylamine, the methylamine:
A) acts as an electrophile
B) acts as a Lewis acid
C) acts as a Bronsted-Lowry acid
D) acts as a Bronsted-Lowry base
E) acts as a nucleophile in an SN2 reaction
A) acts as an electrophile
B) acts as a Lewis acid
C) acts as a Bronsted-Lowry acid
D) acts as a Bronsted-Lowry base
E) acts as a nucleophile in an SN2 reaction

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
11
The structure that corresponds to N-ethyl-2,3-dimethyl-3-hexanamine is
A)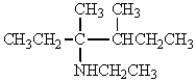
B)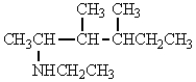
C)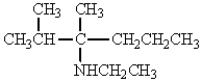
D)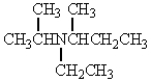
E)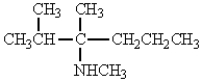
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
12
To separate a mixture of p-toluidine and p-nitrotoluene dissolved in ether,
A) extract the ether solution with aqueous HCl and treat the water layer with aqueous NaOH.
B) extract the ether solution with aqueous NaOH and treat the water layer with aqueous HCl.
C) extract the ether solution with water and treat the water layer with aqueous NaOH.
D) extract the ether layer with aqueous HCl and treat the ether layer with aqueous NaOH.
E) extract the ether solution with aqueous HCl and treat the ether layer with aqueous HCl.
A) extract the ether solution with aqueous HCl and treat the water layer with aqueous NaOH.
B) extract the ether solution with aqueous NaOH and treat the water layer with aqueous HCl.
C) extract the ether solution with water and treat the water layer with aqueous NaOH.
D) extract the ether layer with aqueous HCl and treat the ether layer with aqueous NaOH.
E) extract the ether solution with aqueous HCl and treat the ether layer with aqueous HCl.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
13
Tert-butylamine is classified as a(n) amine.
A) 1°
B) 2°
C) 3°
D) quaternary salt
E) aromatic
A) 1°
B) 2°
C) 3°
D) quaternary salt
E) aromatic

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
14
The alkylation of an amine with an alkyl halide takes place by the following mechanism:
A) SN1
B) SN2
C) electrophilic addition
D) E1
E) E2
A) SN1
B) SN2
C) electrophilic addition
D) E1
E) E2

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
15
The order of decreasing pKas of the corresponding ammonium ions of the following is: 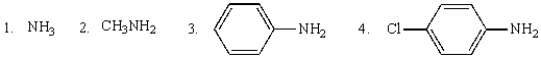
A) 1 > 2 > 3 > 4
B) 3 > 4 > 2 > 1
C) 4 > 3 > 2 > 1
D) 2 > 1 > 3 > 4
E) 4 > 3 > 1 > 2

A) 1 > 2 > 3 > 4
B) 3 > 4 > 2 > 1
C) 4 > 3 > 2 > 1
D) 2 > 1 > 3 > 4
E) 4 > 3 > 1 > 2

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
16
The order of increasing basicity of the following is: 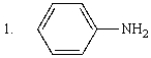
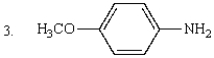
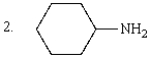
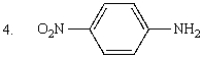
A) 1 < 2 < 3 < 4
B) 2 < 3 < 1 < 4
C) 4 < 3 < 2 < 1
D) 4 < 3 < 1 < 2
E) 4 < 1 < 3 < 2




A) 1 < 2 < 3 < 4
B) 2 < 3 < 1 < 4
C) 4 < 3 < 2 < 1
D) 4 < 3 < 1 < 2
E) 4 < 1 < 3 < 2

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
17
The order of decreasing acidity of the following is: 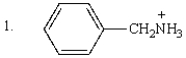
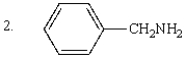
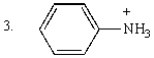
A) 1 > 2 > 3
B) 3 > 1 > 2
C) 2 > 1 > 3
D) 3 > 2 > 1
E) 2 > 3 > 1



A) 1 > 2 > 3
B) 3 > 1 > 2
C) 2 > 1 > 3
D) 3 > 2 > 1
E) 2 > 3 > 1

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
18
What structure represents a cationic intermediate in the electrophilic aromatic bromination of aniline? 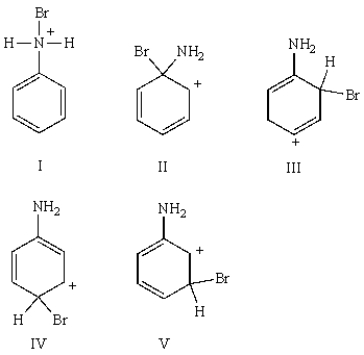
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following molecules has the highest boiling point?
A) ethylamine
B) propane
C) ethyl alcohol
D) dimethyl ether
E) acetaldehyde
A) ethylamine
B) propane
C) ethyl alcohol
D) dimethyl ether
E) acetaldehyde

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following statements is false:
A) Aliphatic 3o amines with three different groups on nitrogen can be resolved.
B) Primary and secondary aliphatic amines can be hydrogen bond donors.
C) Aliphatic amines can be hydrogen bond acceptors.
D) The non-bonded lone pair in an aliphatic amine is more basic than the non-bonded lone pairs in ethers.
E) The nitrogen in aliphatic amines is sp3-hybridized.
A) Aliphatic 3o amines with three different groups on nitrogen can be resolved.
B) Primary and secondary aliphatic amines can be hydrogen bond donors.
C) Aliphatic amines can be hydrogen bond acceptors.
D) The non-bonded lone pair in an aliphatic amine is more basic than the non-bonded lone pairs in ethers.
E) The nitrogen in aliphatic amines is sp3-hybridized.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
21
What type of products are formed by diazo coupling reactions?
A) meso compounds
B) azo compounds
C) diazonium salts
D) quaternary ammonium salts
E) racemic mixtures
A) meso compounds
B) azo compounds
C) diazonium salts
D) quaternary ammonium salts
E) racemic mixtures

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
22
Aniline 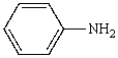 is best prepared via:
is best prepared via:
A)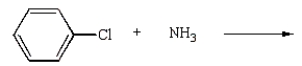
B)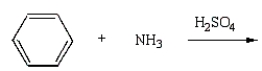
C)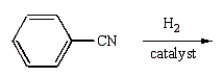
D)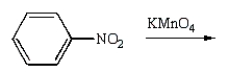
E)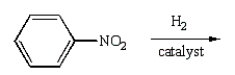
 is best prepared via:
is best prepared via:A)
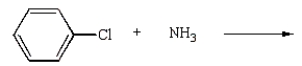
B)
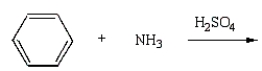
C)

D)

E)


Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
23
What reacts with benzenediazonium chloride to produce benzonitrile?
A) HONO
B) Li, NH3
C) NaBH3CN
D) KCN, Cu2CN2
E) LiAlH4, ether
A) HONO
B) Li, NH3
C) NaBH3CN
D) KCN, Cu2CN2
E) LiAlH4, ether

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
24
What is the name of the product formed by reacting CuCl and HCl with benzenediazonium chloride?
A) bromobenzene
B) chlorobenzene
C) o-chloroaniline
D) m-chloroaniline
E) p-chloroaniline
A) bromobenzene
B) chlorobenzene
C) o-chloroaniline
D) m-chloroaniline
E) p-chloroaniline

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
25
The reaction of a 2o amine with nitrous acid gives:
A) a quaternary ammonium salt
B) a nitrosamine
C) A diazonium salt
D) an azo dye
E) a nitro compound
A) a quaternary ammonium salt
B) a nitrosamine
C) A diazonium salt
D) an azo dye
E) a nitro compound

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
26
Reacting aniline, C6H5NH2, with a primary alkyl halide will produce a(n):
A) 1° amine
B) 2° amine
C) 3° amine
D) amide
E) imine
A) 1° amine
B) 2° amine
C) 3° amine
D) amide
E) imine

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
27
The product(s) of 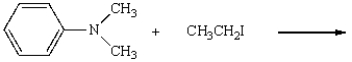 are:
are:
A)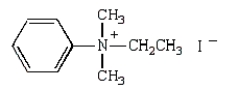
B)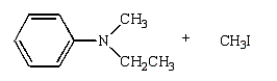
C)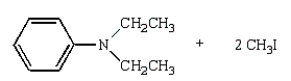
D)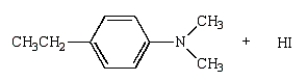
E)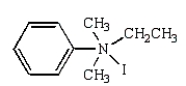
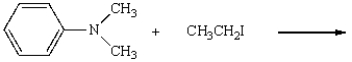 are:
are:A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
28
m-Dichlorobenzene can be prepared in good yield by the sequence:
A)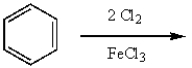
B)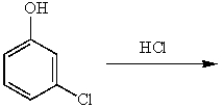
C)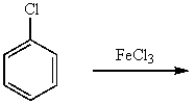
D)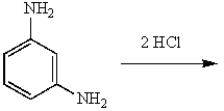
E)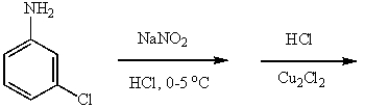
A)
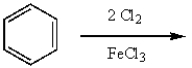
B)

C)

D)

E)


Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
29
What is the final product for the following sequence of reactions?
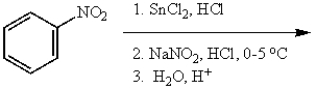
A)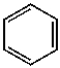
B)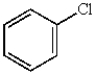
C)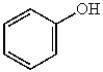
D)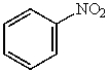
E)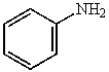
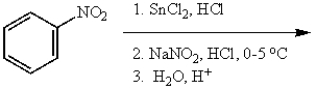
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
30
What is the stereochemical relationship of the products formed by reacting racemic lactic acid with (S)-1-phenylethylamine?
A) enantiomers
B) meso compounds
C) racemic mixture
D) diastereomers
E) geometric isomers
A) enantiomers
B) meso compounds
C) racemic mixture
D) diastereomers
E) geometric isomers

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
31
The amines that can be acylated by acetic anhydride are 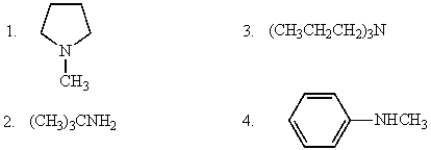
A) only 4
B) 1, 2, 3, and 4
C) 1 and 3
D) 2 and 4
E) only 2

A) only 4
B) 1, 2, 3, and 4
C) 1 and 3
D) 2 and 4
E) only 2

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
32
What is the name of the product of the following reaction? 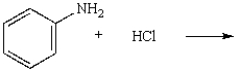
A) chlorobenzene
B) chloroaniline
C) anilinium chloride
D) o-chloroaniline
E) N-chloroaniline
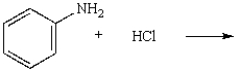
A) chlorobenzene
B) chloroaniline
C) anilinium chloride
D) o-chloroaniline
E) N-chloroaniline

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
33
The following reaction sequence will produce: 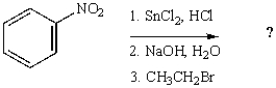
A)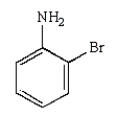
B)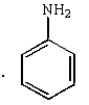
C)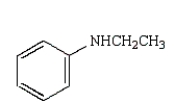
D)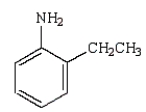
E)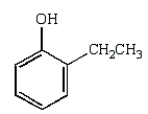
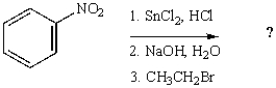
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
34
Which molecule is known as an azo compound?
A)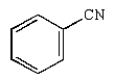
B)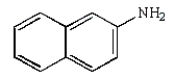
C)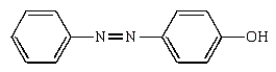
D)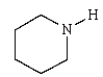
E)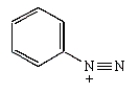
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
35
An imine is produced when a ketone or aldehyde reacts with:
A) methyl alcohol
B) Zn(Hg), HCl
C) an amine
D) an amide
E) HCN
A) methyl alcohol
B) Zn(Hg), HCl
C) an amine
D) an amide
E) HCN

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
36
The product(s) from the reaction sequence below is (are): 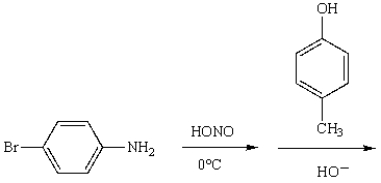
A)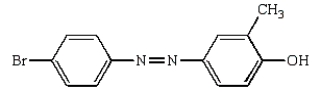
B)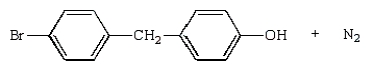
C)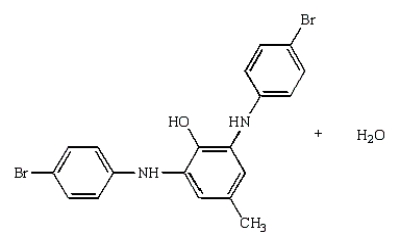
D)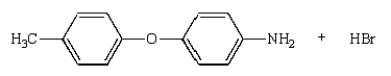
E)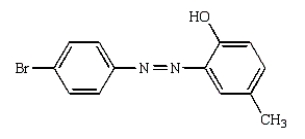

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
37
The product(s) from the reaction below are: 
A)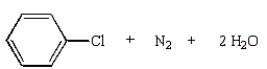
B)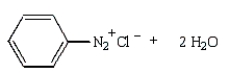
C)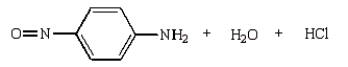
D)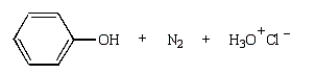
E)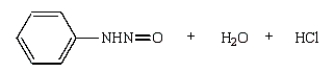

A)
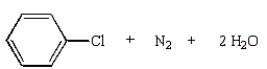
B)
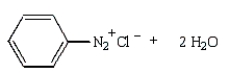
C)
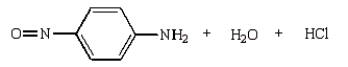
D)
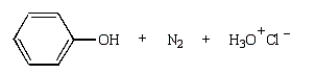
E)
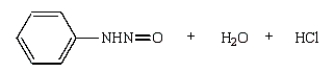

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
38
m-Chlorobromobenzene can be prepared in good yield by the sequence:
A)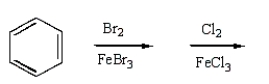
B)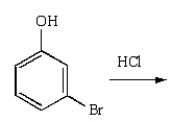
C)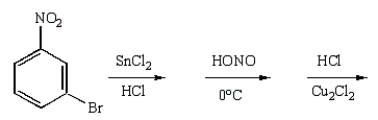
D)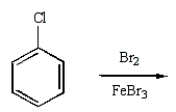
E)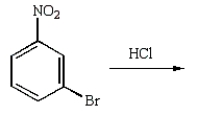
A)
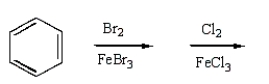
B)

C)

D)

E)


Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
39
Which reaction will produce a quaternary ammonium salt?
A)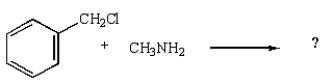
B)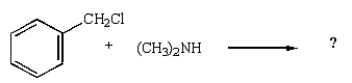
C)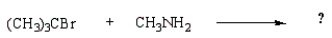
D)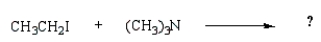
E)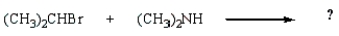
A)
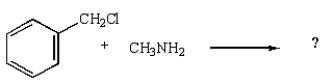
B)
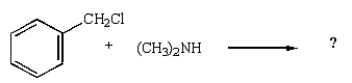
C)
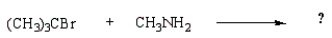
D)
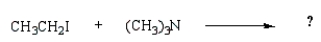
E)
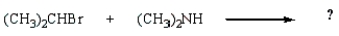

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following amines can be converted to an aryl diazonium salt?
A)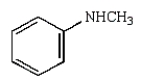
B)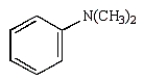
C)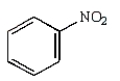
D)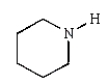
E)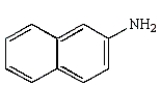
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck



