Deck 9: Aldehydes and Ketones
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/45
Play
Full screen (f)
Deck 9: Aldehydes and Ketones
1
Which of the hydrogens in the following molecule are most acidic? The hydrogens on carbon 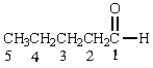
A) 1
B) 2
C) 3
D) 4
E) 5
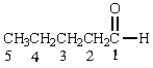
A) 1
B) 2
C) 3
D) 4
E) 5
2
2
What is the structure of cyclohexanecarbaldehyde?
A)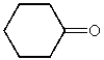
B)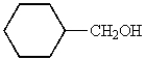
C)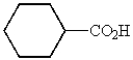
D)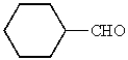
E)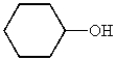
A)

B)

C)

D)

E)


3
The enol tautomer of 3-pentanone is:
A)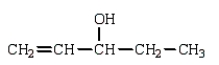
B)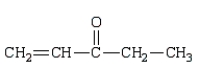
C)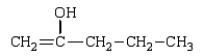
D)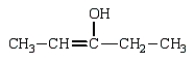
E)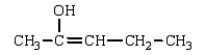
A)

B)

C)
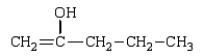
D)

E)
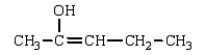

4
What class of compound most closely resembles an acetal in its reactivity with CH3MgBr?
A) ethers
B) aldehydes
C) ketones
D) alcohols
E) thiols
A) ethers
B) aldehydes
C) ketones
D) alcohols
E) thiols

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following compounds is a cyanohydrin?
A)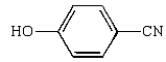
B)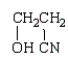
C)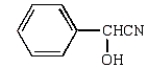
D)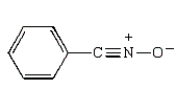
E)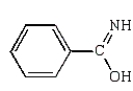
A)

B)
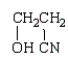
C)

D)

E)


Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following compounds is a hemiacetal?
A)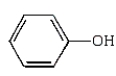
B)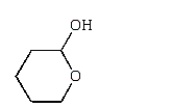
C)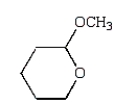
D)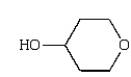
E)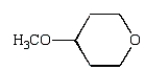
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
7
The number of a-hydrogens in 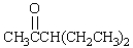 is:
is:
A) 1
B) 3
C) 4
D) 8
E) 14
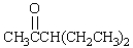 is:
is:A) 1
B) 3
C) 4
D) 8
E) 14

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following molecules is a hemiacetal?
A)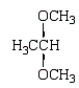
B)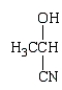
C)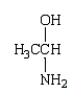
D)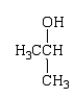
E)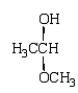
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following aldehydes can exist in equilibrium with a cyclic hemiacetal?
A) 4-pentenal
B) 3-hydroxypropanal
C) 2-hydroxybutanal
D) 3-hydroxybutanal
E) 4-hydroxybutanal
A) 4-pentenal
B) 3-hydroxypropanal
C) 2-hydroxybutanal
D) 3-hydroxybutanal
E) 4-hydroxybutanal

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
10
The equilibrium that exists between the keto and enol forms of aldehydes and ketones is known as:
A) stereoisomerism
B) configurational isomerism
C) geometric isomerism
D) tautomerism
E) positional isomerism
A) stereoisomerism
B) configurational isomerism
C) geometric isomerism
D) tautomerism
E) positional isomerism

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
11
The name of the following is:
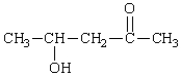
A) 2-hydroxy-4-pentanone.
B) 2-oxo-4-pentanol.
C) 4-oxo-2-pentanol.
D) 1-acetyl-2-propanol.
E) 4-hydroxy-2-pentanone.
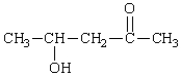
A) 2-hydroxy-4-pentanone.
B) 2-oxo-4-pentanol.
C) 4-oxo-2-pentanol.
D) 1-acetyl-2-propanol.
E) 4-hydroxy-2-pentanone.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
12
The expected order of boiling points of the following is: 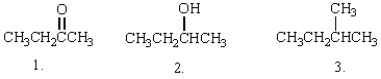
A) 3>2>1
B) 2>1>3
C) 2>3>1
D) 1>2>3
E) 1>3>2
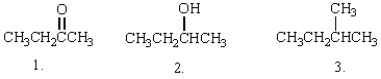
A) 3>2>1
B) 2>1>3
C) 2>3>1
D) 1>2>3
E) 1>3>2

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
13
The predominant product from the reaction below is: 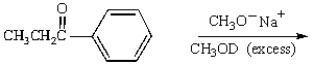
A)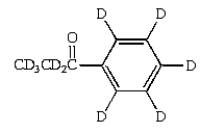
B)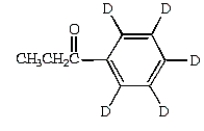
C)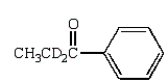
D)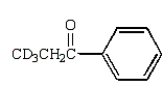
E)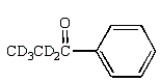

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
14
The IUPAC name for ethyl methyl ketone is
A) 2-butanone
B) 2-pentanone
C) 3-pentanone
D) propanone
E) acetophenone
A) 2-butanone
B) 2-pentanone
C) 3-pentanone
D) propanone
E) acetophenone

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following molecules has the highest boiling point?
A) o-xylene
B) m-xylene
C) p-xylene
D) benzaldehyde
E) benzyl alcohol
A) o-xylene
B) m-xylene
C) p-xylene
D) benzaldehyde
E) benzyl alcohol

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
16
The IUPAC name for the following molecule is: 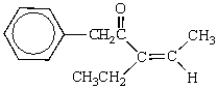
A) 3-ethyl-1-phenyl-3-pentenone
B) 3-ethyl-5-phenyl-2-penten-4-one
C) allyl benzyl ketone
D) (E)-3-ethyl-1-phenyl-3-penten-2-one
E) (Z)-3-ethyl-1-phenyl-3-penten-2-one

A) 3-ethyl-1-phenyl-3-pentenone
B) 3-ethyl-5-phenyl-2-penten-4-one
C) allyl benzyl ketone
D) (E)-3-ethyl-1-phenyl-3-penten-2-one
E) (Z)-3-ethyl-1-phenyl-3-penten-2-one

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
17
What is the class of compound produced from the reaction of a ketone with hydrazine?
A) oxime
B) amide
C) hydrazone
D) semicarbazone
E) imide
A) oxime
B) amide
C) hydrazone
D) semicarbazone
E) imide

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
18
The common name for the following molecule is: 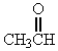
A) acetaldehyde
B) dimethyl aldehyde
C) ethyl ketone
D) formaldehyde
E) methylmethanal
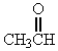
A) acetaldehyde
B) dimethyl aldehyde
C) ethyl ketone
D) formaldehyde
E) methylmethanal

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is a hydrazone?
A)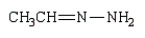
B)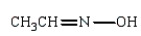
C)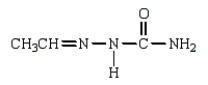
D)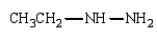
E)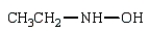
A)
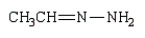
B)
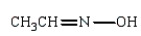
C)

D)
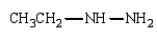
E)
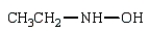

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
20
In a carbonyl group
A) the oxygen acts as a Lewis acid.
B) the C=O bond length is shortened due to resonance.
C) the carbon is sp3 hybridized.
D) the carbon is nucleophilic and the oxygen is electrophilic.
E) the carbon is electrophilic and the oxygen is nucleophilic.
A) the oxygen acts as a Lewis acid.
B) the C=O bond length is shortened due to resonance.
C) the carbon is sp3 hybridized.
D) the carbon is nucleophilic and the oxygen is electrophilic.
E) the carbon is electrophilic and the oxygen is nucleophilic.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
21
What type of compound is produced from the following reaction? 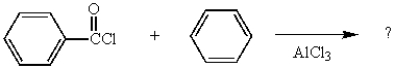
A) an amide
B) an alcohol
C) an acid
D) an aldehyde
E) a ketone

A) an amide
B) an alcohol
C) an acid
D) an aldehyde
E) a ketone

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following nucleophiles add reversibly to a carbonyl group? 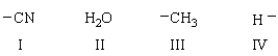
A) I, II and IV
B) II
C) I and II
D) III and IV
E) I, II, III and IV
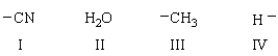
A) I, II and IV
B) II
C) I and II
D) III and IV
E) I, II, III and IV

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
23
Which hydrogens in the following compound will be exchanged most rapidly for deuterium upon reaction with D2O and NaOD? 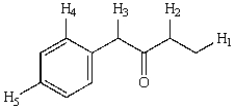
A) H1
B) H2
C) H3
D) H4
E) H5

A) H1
B) H2
C) H3
D) H4
E) H5

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
24
In the mechanism for acid catalyzed hemiacetal formation, the first step is:
A) protonation of the carbonyl oxygen
B) nucleophilic attack at the carbonyl carbon
C) protonation of the oxygen of the alcohol
D) nucleophilic attack at the carbon of the alcohol
E) elimination of a water molecule
A) protonation of the carbonyl oxygen
B) nucleophilic attack at the carbonyl carbon
C) protonation of the oxygen of the alcohol
D) nucleophilic attack at the carbon of the alcohol
E) elimination of a water molecule

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following compounds will not act as a nucleophile in an Aldol reaction?
A)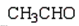
B)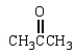
C)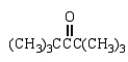
D) HCHO
E) C and D
A)
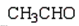
B)
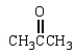
C)
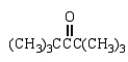
D) HCHO
E) C and D

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
26
What statement is false relative to the nucleophilic additions?
A) When a weak nucleophile is present, the reaction can be catalyzed by acid.
B) The nucleophile attacks the trigonal carbon of the carbonyl group.
C) Ketones are more reactive than aldehydes.
D) Nucleophiles that add irreversibly are poor leaving groups.
E) Nucleophiles can be classified as those that add reversibly to carbonyl compounds and those that add irreversibly.
A) When a weak nucleophile is present, the reaction can be catalyzed by acid.
B) The nucleophile attacks the trigonal carbon of the carbonyl group.
C) Ketones are more reactive than aldehydes.
D) Nucleophiles that add irreversibly are poor leaving groups.
E) Nucleophiles can be classified as those that add reversibly to carbonyl compounds and those that add irreversibly.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
27
Which statement about the mechanism of imine formation from a primary amine and an aldehyde or ketone is false?
A) The first steps involve addition of the amine to the carbonyl carbon to form a tetrahedral intermediate.
B) The last steps involve elimination of water to form a carbon-nitrogen p-bond.
C) All steps are reversible.
D) The reaction involves SN2 displacement of the carbonyl oxygen by the amine nitrogen.
E) The reaction does not require a strong acid catalyst.
A) The first steps involve addition of the amine to the carbonyl carbon to form a tetrahedral intermediate.
B) The last steps involve elimination of water to form a carbon-nitrogen p-bond.
C) All steps are reversible.
D) The reaction involves SN2 displacement of the carbonyl oxygen by the amine nitrogen.
E) The reaction does not require a strong acid catalyst.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following reactions will produce a cyanohydrin?
A)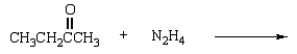
B)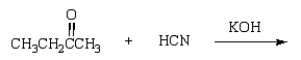
C)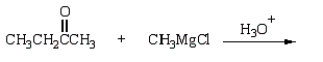
D)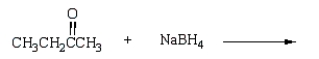
E)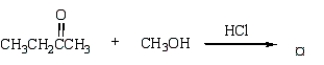
A)
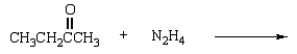
B)
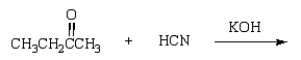
C)
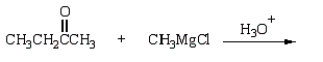
D)
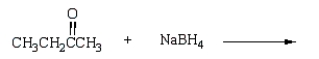
E)


Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
29
The products from 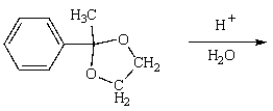 are:
are:
A)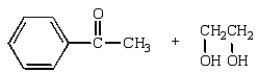
B)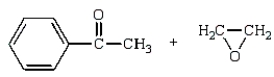
C)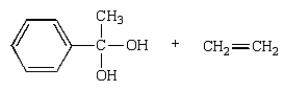
D)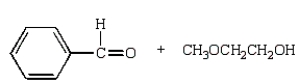
E) no reaction
 are:
are:A)
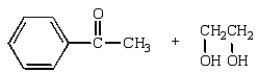
B)
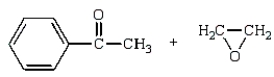
C)
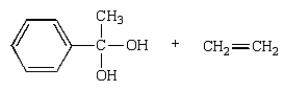
D)
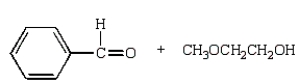
E) no reaction

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following compounds will give a positive silver mirror test (Tollens' test)?
A)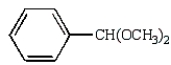
B)
C)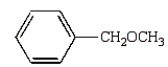
D)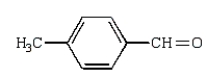
E)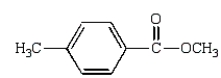
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
31
What is the structure of the aldol produced from reacting propanone with NaOH?
A)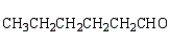
B)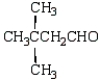
C)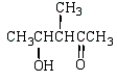
D)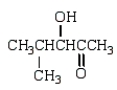
E)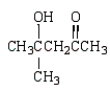
A)
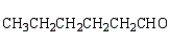
B)

C)

D)

E)
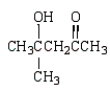

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
32
The second step in the base catalyzed aldol condensation is:
A) formation of the enolate ion
B) addition of an enolate to a carbonyl group
C) protonation of the alkoxide ion
D) protonation of the carbonyl oxygen
E) loss of a proton from the carbon
A) formation of the enolate ion
B) addition of an enolate to a carbonyl group
C) protonation of the alkoxide ion
D) protonation of the carbonyl oxygen
E) loss of a proton from the carbon

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
33
Which reagent will accomplish the following transformation? 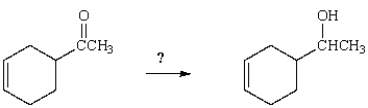
A) NaOH
B) CrO3, H2SO4
C) CH3MgBr
D) NaBH4
E) H2, Pd

A) NaOH
B) CrO3, H2SO4
C) CH3MgBr
D) NaBH4
E) H2, Pd

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
34
What reaction will produce the following product? 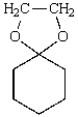
A)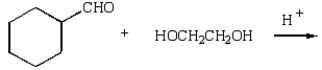
B)
C)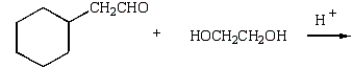
D)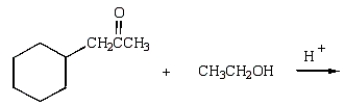
E)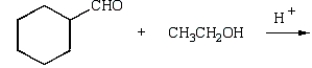

A)

B)

C)
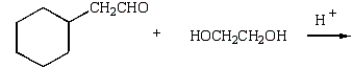
D)
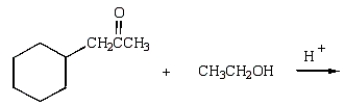
E)
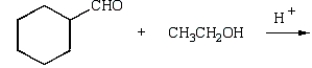

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
35
What Grignard reagent and carbonyl compound react to give benzyl alcohol after treatment with aqueous acid?
A) phenyl magnesium bromide and formaldehyde
B) phenyl magnesium bromide and oxirane
C) benzaldehyde and methyl magnesium bromide
D) benzaldehyde and ethyl magnesium chloride
E) acetophenone and methyl magnesium chloride
A) phenyl magnesium bromide and formaldehyde
B) phenyl magnesium bromide and oxirane
C) benzaldehyde and methyl magnesium bromide
D) benzaldehyde and ethyl magnesium chloride
E) acetophenone and methyl magnesium chloride

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
36
Which reagents would you use to accomplish the following conversion? 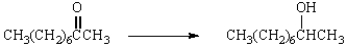
A) NaBH4, H2O
B) LiAlH4, ether; then H3O+
C) H2, Pt
D) all of these
E) none of these

A) NaBH4, H2O
B) LiAlH4, ether; then H3O+
C) H2, Pt
D) all of these
E) none of these

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
37
An oxime can be produced by the reaction of an aldehyde and:
A) hydroxylamine
B) hydrazine
C) methylamine
D) phenylhydrazine
E) semicarbazide
A) hydroxylamine
B) hydrazine
C) methylamine
D) phenylhydrazine
E) semicarbazide

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
38
The product from 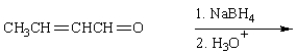 is:
is:
A)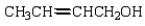
B)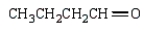
C)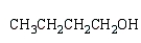
D)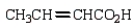
E)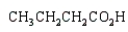
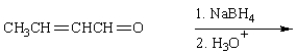 is:
is:A)
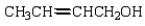
B)
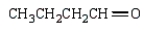
C)
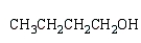
D)
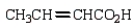
E)
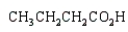

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
39
The reaction of a Grignard reagent with acetone followed by acid hydrolysis will produce what type of product?
A) a primary alcohol
B) a secondary alcohol
C) a tertiary alcohol
D) a carboxylic acid
E) a ketone
A) a primary alcohol
B) a secondary alcohol
C) a tertiary alcohol
D) a carboxylic acid
E) a ketone

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
40
What reactants give the following molecule upon acid hydrolysis? 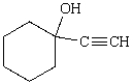
A) cyclohexyl magnesium bromide and acetaldehyde
B) cyclohexanol and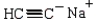
C) cyclohexanone and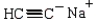
D) cyclohexanecarbaldehyde and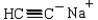
E) cyclohexanone and ethenyl magnesium bromide

A) cyclohexyl magnesium bromide and acetaldehyde
B) cyclohexanol and
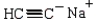
C) cyclohexanone and
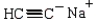
D) cyclohexanecarbaldehyde and
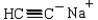
E) cyclohexanone and ethenyl magnesium bromide

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
41
The aldol obtained by treating 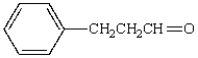 with base is:
with base is:
A)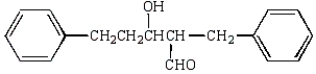
B)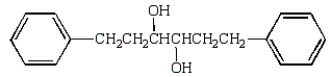
C)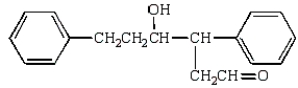
D)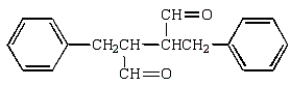
E)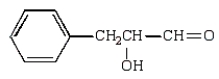
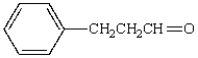 with base is:
with base is:A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
42
The organic product of the reaction 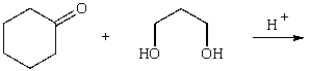 is:
is:
A)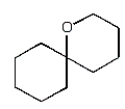
B)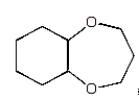
C)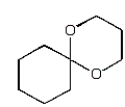
D)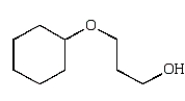
E)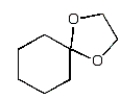
 is:
is:A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
43
How many hydrogens in the following compound will be exchanged for deuterium upon reaction with D2O and an acid catalyst? 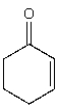
A) 0
B) 2
C) 4
D) 5
E) 8

A) 0
B) 2
C) 4
D) 5
E) 8

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
44
The major product obtained from 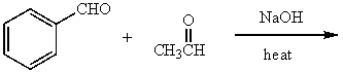 is:
is:
A)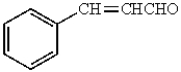
B)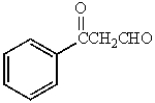
C)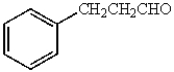
D)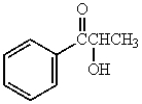
E)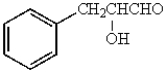
 is:
is:A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
45
Which reagent can be used to accomplish the following transformation? 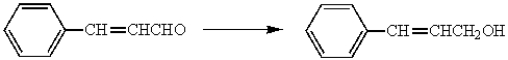
A) pyridinium chlorochromate
B) Tollens' reagent
C) NaBH4
D) H2, Ni
E) NaH

A) pyridinium chlorochromate
B) Tollens' reagent
C) NaBH4
D) H2, Ni
E) NaH

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck



