Deck 6: Organic Halogen Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/34
Play
Full screen (f)
Deck 6: Organic Halogen Compounds
1
Which of the following is an incorrect representation of relative nucleophile strength?
A) (-NH2 > F-)
B) HO- > HS-
C) CH3- > HO-
D) CH3O- > CH3OH
E) I- > Br-
A) (-NH2 > F-)
B) HO- > HS-
C) CH3- > HO-
D) CH3O- > CH3OH
E) I- > Br-
HO- > HS-
2
Which of the following is the best nucleophile?
A) H2O
B) HO-
C) HS-
D) H3S
E) all are the same
A) H2O
B) HO-
C) HS-
D) H3S
E) all are the same
HS-
3
The slowest step of an SN1 reaction involves:
A) attack of the nucleophile on the substrate to form a pentavalent carbon.
B) breaking the bond between the carbon and the leaving group to give a carbocation.
C) combination of a nucleophile with the carbocation to give the product.
D) loss of a proton from the nucleophile to give the product.
E) none of the above.
A) attack of the nucleophile on the substrate to form a pentavalent carbon.
B) breaking the bond between the carbon and the leaving group to give a carbocation.
C) combination of a nucleophile with the carbocation to give the product.
D) loss of a proton from the nucleophile to give the product.
E) none of the above.
breaking the bond between the carbon and the leaving group to give a carbocation.
4
Which of the following is the best leaving group?
A) F-
B) Cl-
C) I-
D) Br-
E) H2N-
A) F-
B) Cl-
C) I-
D) Br-
E) H2N-

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following bromides will react faster with CH3OH in an SN1 reaction?
A)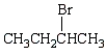
B)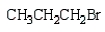
C)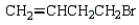
D)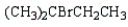
E)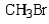
A)
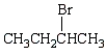
B)
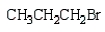
C)
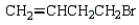
D)
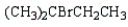
E)
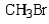

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is the best nucleophile?
A) H2O
B) CH4
C) NH3
D) HF
A) H2O
B) CH4
C) NH3
D) HF

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is the strongest base?
A) H2O
B) (-OH)
C) NH3
D) (-NH2)
E) F-
A) H2O
B) (-OH)
C) NH3
D) (-NH2)
E) F-

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
8
Which statement is true for SN2 reactions?
A) The rate of the reaction is dependent on the stability of a carbocation.
B) The rate of reaction is dependent on just the substrate.
C) The fastest reaction will occur with a tertiary halide.
D) Displacement occurs with inversion of configuration.
E) The mechanism is a two step process.
A) The rate of the reaction is dependent on the stability of a carbocation.
B) The rate of reaction is dependent on just the substrate.
C) The fastest reaction will occur with a tertiary halide.
D) Displacement occurs with inversion of configuration.
E) The mechanism is a two step process.

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
9
What is the mechanism of the following reaction? 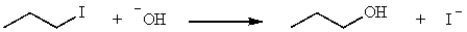
A) SN1
B) SN2
C) E1
D) E2
E) both A and B
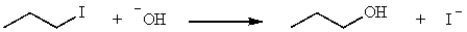
A) SN1
B) SN2
C) E1
D) E2
E) both A and B

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
10
CH3CH2C(CH3)2Br + (CH3)3CO-K+ are most likely to react by
A) a free radical chain mechanism.
B) the SN1 mechanism.
C) the SN2 mechanism.
D) the E1 mechanism.
E) the E2 mechanism.
A) a free radical chain mechanism.
B) the SN1 mechanism.
C) the SN2 mechanism.
D) the E1 mechanism.
E) the E2 mechanism.

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
11
The expected SN2 reactivity order of the following is: 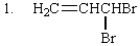
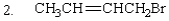
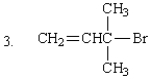
A) 2 > 1 > 3
B) 2 > 3 > 1
C) 1 > 2 > 3
D) 1 > 3 > 2
E) 3 > 1 > 2
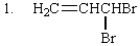
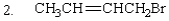

A) 2 > 1 > 3
B) 2 > 3 > 1
C) 1 > 2 > 3
D) 1 > 3 > 2
E) 3 > 1 > 2

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
12
The SN2 mechanism for nucleophilic substitution reactions
A) involves two steps and occurs with inversion of configuration.
B) involves one step and occurs with inversion of configuration.
C) involves two steps and occurs with racemization.
D) involves one step and occurs with retention of configuration.
E) involves one step and occurs with racemization.
A) involves two steps and occurs with inversion of configuration.
B) involves one step and occurs with inversion of configuration.
C) involves two steps and occurs with racemization.
D) involves one step and occurs with retention of configuration.
E) involves one step and occurs with racemization.

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
13
The SN1 mechanism for nucleophilic substitution reactions
A) involves one step and occurs fastest with primary halides.
B) involves one step and occurs fastest with tertiary halides.
C) involves two steps and occurs fastest with tertiary halides.
D) involves two steps and occurs fastest with primary halides.
E) involves one step and occurs fastest with aromatic halides.
A) involves one step and occurs fastest with primary halides.
B) involves one step and occurs fastest with tertiary halides.
C) involves two steps and occurs fastest with tertiary halides.
D) involves two steps and occurs fastest with primary halides.
E) involves one step and occurs fastest with aromatic halides.

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
14
What is the IUPAC name of the following alkyl halide? 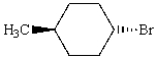
A) trans-p-bromotoluene
B) trans-4-methylcyclohexyl bromide
C) trans-4-methyl-1-bromocyclohexane
D) trans-1-bromo-4-methylcyclohexane
E) trans-p-bromomethylcyclohexane

A) trans-p-bromotoluene
B) trans-4-methylcyclohexyl bromide
C) trans-4-methyl-1-bromocyclohexane
D) trans-1-bromo-4-methylcyclohexane
E) trans-p-bromomethylcyclohexane

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
15
When (R)-3-bromo-3-methylhexane is treated with H2O, racemic is produced.By what mechanism does this reaction occur? 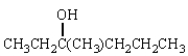
A) SN1
B) SN2
C) E1
D) E2
E) cannot be explained by one mechanism
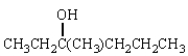
A) SN1
B) SN2
C) E1
D) E2
E) cannot be explained by one mechanism

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
16
Which statement(s) is/are true of an E1 elimination?
A) it is a two-step process and has the same first step as a SN1 mechanism
B) it involves the formation of the carbocation from elimination of a good leaving group
C) a common competing reaction is rearrangement of a less stable carbocation to a more stable carbocation
D) the loss of a proton by the carbocation is a fast step
E) all of the above
A) it is a two-step process and has the same first step as a SN1 mechanism
B) it involves the formation of the carbocation from elimination of a good leaving group
C) a common competing reaction is rearrangement of a less stable carbocation to a more stable carbocation
D) the loss of a proton by the carbocation is a fast step
E) all of the above

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following structures represents 2,4,4-trichloroheptane? 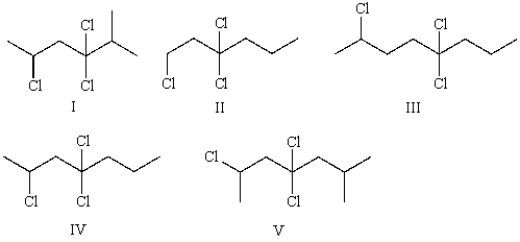
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
18
Which bromide reacts fastest in SN2 reactions?
A) CH3Br
B) (CH3) 2CHBr
C) (CH3) 3CBr
D) (CH3) 3CCH2Br
E) CH3CH2Br
A) CH3Br
B) (CH3) 2CHBr
C) (CH3) 3CBr
D) (CH3) 3CCH2Br
E) CH3CH2Br

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is a polar aprotic solvent?
A) H2O
B) CH3CN
C) CH3OH
D) (CH3) 2S=O
E) both B and D
A) H2O
B) CH3CN
C) CH3OH
D) (CH3) 2S=O
E) both B and D

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
20
What is the IUPAC name of the following alkyl halide? 
A) 2-bromo-6-methylheptane
B) 6-methyl-2-bromoheptane
C) 2-methyl-6-bromoheptane
D) 2-bromo-2-methylheptane
E) 2-bromo-5-isopropylpentane

A) 2-bromo-6-methylheptane
B) 6-methyl-2-bromoheptane
C) 2-methyl-6-bromoheptane
D) 2-bromo-2-methylheptane
E) 2-bromo-5-isopropylpentane

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
21
The major product of the reaction below is: 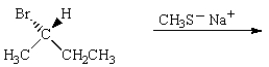 is
is
A)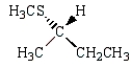
B)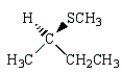
C)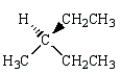
D)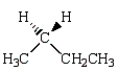
E)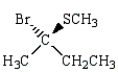
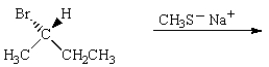 is
isA)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
22
What is the final product of the following sequence of reactions? 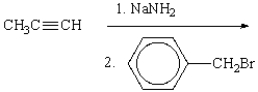
A) I
B) II
C) III
D) IV
E) III and IV


A) I
B) II
C) III
D) IV
E) III and IV

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
23
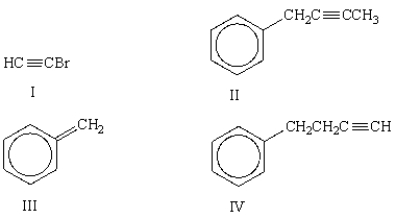
What is the major product of the following reaction? 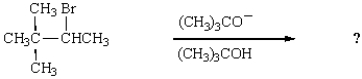
A)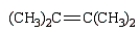
B)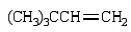
C)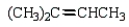
D)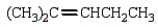
E) none of these
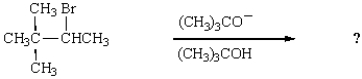
A)
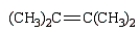
B)
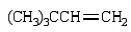
C)
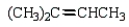
D)
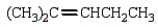
E) none of these

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
24
The structure below represents the transition state for the reaction of 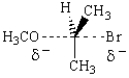
A) methanol with 2-bromopropene.
B) methoxide with 2-bromopropane.
C) sodium bromide with isopropyl methyl ether.
D) methanol with 2-bromopropane.
E) methoxide with 1-bromopropane.

A) methanol with 2-bromopropene.
B) methoxide with 2-bromopropane.
C) sodium bromide with isopropyl methyl ether.
D) methanol with 2-bromopropane.
E) methoxide with 1-bromopropane.

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
25
The major product of the following reaction is: 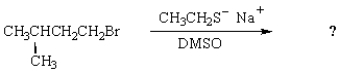
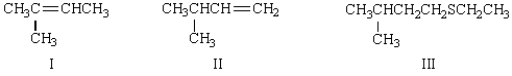
A) I
B) II
C) III
D) I and II
E) there is no major product

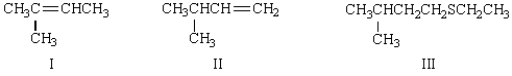
A) I
B) II
C) III
D) I and II
E) there is no major product

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
26
Polyhalogenated aliphatic compounds have not been used as:
A) food preservatives
B) fire retardants
C) degreasing agents
D) insecticides
E) refrigerants
A) food preservatives
B) fire retardants
C) degreasing agents
D) insecticides
E) refrigerants

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
27
What is the major product of the following reaction? 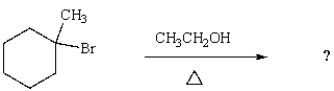
A)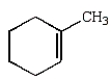
B)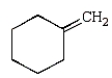
C)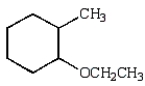
D)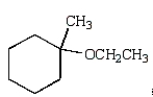
E)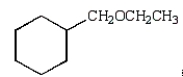
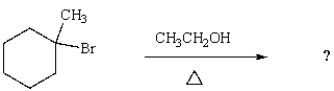
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
28
Which Fischer projection represents the product of the following reaction? 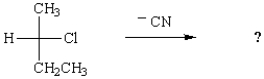
A)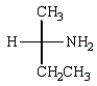
B)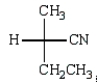
C)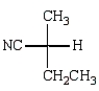
D)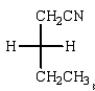
E)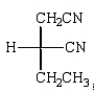
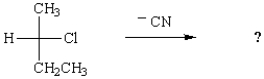
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
29
When 1-bromobutane is reacted with the bulky base, potassium tert-butoxide, in tert-butyl alcohol, the major elimination product is:
A) 1-butene
B) cis-2-butene
C) trans-2-butene
D) butyl tert-butyl ether
E) butyl alcohol
A) 1-butene
B) cis-2-butene
C) trans-2-butene
D) butyl tert-butyl ether
E) butyl alcohol

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
30
The monomer used to prepare Teflon is
A) CH2=CH2
B) CH2=CHCl
C) CH3CH=CH2
D) CF2=CF2
E) CH2Cl2
A) CH2=CH2
B) CH2=CHCl
C) CH3CH=CH2
D) CF2=CF2
E) CH2Cl2

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
31
The products of the following reactions are: 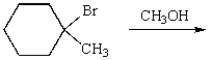
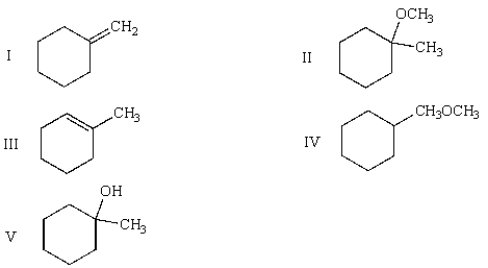
A) I, III
B) I, II, III
C) III, V
D) II, IV, V
E) II, III
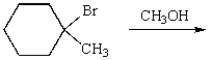

A) I, III
B) I, II, III
C) III, V
D) II, IV, V
E) II, III

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
32
How many different E2 products can form from the dehydrohalogenation of 2-bromopentane?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
33
What alkyne is produced when sodium acetylide reacts with CH3CH2CH2I?
A) 2-pentyne
B) 1-pentyne
C) 2-pentene
D) 1-pentene
E) butyne
A) 2-pentyne
B) 1-pentyne
C) 2-pentene
D) 1-pentene
E) butyne

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following halocarbons is the raw material for the synthesis of Teflon? 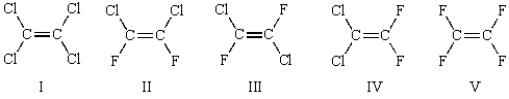
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck



