Deck 4: Aromatic Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/46
Play
Full screen (f)
Deck 4: Aromatic Compounds
1
Which of the following structures accurately represents phenol?
A)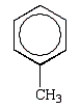
B)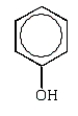
C)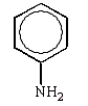
D)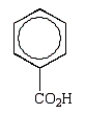
E)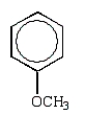
A)

B)

C)

D)

E)


2
How many different trisubstituted products are possible from the nitration of o-xylene?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5
2
3
Using the following monosubstituted benzene, which position would be meta to X? 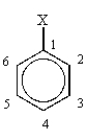
A) 1
B) 2
C) 3
D) 4
E) 5

A) 1
B) 2
C) 3
D) 4
E) 5
3
4
What dibromobenzene can form only one tribromobenzene?
A) o-dibromobenzene
B) m-dibromobenzene
C) p-dibromobenzene
D) cumene
E) styrene
A) o-dibromobenzene
B) m-dibromobenzene
C) p-dibromobenzene
D) cumene
E) styrene

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following names represents more than one compound?
A) dichlorobenzene
B) 2-bromophenol
C) o-nitrobenzaldehyde
D) 2,4,6-trinitrotoluene
E) cumene
A) dichlorobenzene
B) 2-bromophenol
C) o-nitrobenzaldehyde
D) 2,4,6-trinitrotoluene
E) cumene

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
6
Which name(s) of the following molecule is/are incorrect? 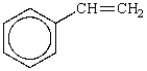
A) styrene
B) vinylbenzene
C) ethylbenzene
D) phenylethene
E) a and b

A) styrene
B) vinylbenzene
C) ethylbenzene
D) phenylethene
E) a and b

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following molecules is o-nitrophenol?
A)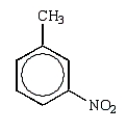
B)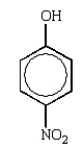
C)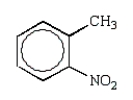
D)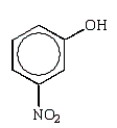
E)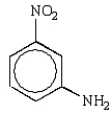
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
8
Which statement about benzene is true?
A) All six hydrogens in benzene are chemically equivalent.
B) Benzene decolorizes bromine solutions.
C) The molecule is planar, and each carbon is at the corner of a regular hexagon.
D) Both a and c are true.
E) Both b and c are true.
A) All six hydrogens in benzene are chemically equivalent.
B) Benzene decolorizes bromine solutions.
C) The molecule is planar, and each carbon is at the corner of a regular hexagon.
D) Both a and c are true.
E) Both b and c are true.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following statements about benzene is FALSE?
A) the molecule is planar and each carbon is at a corner of regular hexagon
B) there are two resonance structures of equivalent energy
C) the bond angles are all 120º and the bond lengths are all 1.39Å
D) the typical mechanism by which reactions occur is by addition
E) each carbon in the benzene ring is sp2 hybridized
A) the molecule is planar and each carbon is at a corner of regular hexagon
B) there are two resonance structures of equivalent energy
C) the bond angles are all 120º and the bond lengths are all 1.39Å
D) the typical mechanism by which reactions occur is by addition
E) each carbon in the benzene ring is sp2 hybridized

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is NOT an electrophile in an electrophilic aromatic substitution reaction?
A) NO2+
B) (CH3)3C+
C) SO3
D) Cl-
E) all are
A) NO2+
B) (CH3)3C+
C) SO3
D) Cl-
E) all are

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
11
Which alkylbenzene, C9H12, when nitrated can yield only one mononitro product?
A)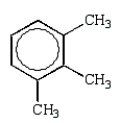
B)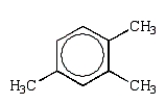
C)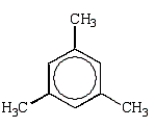
D)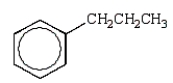
E)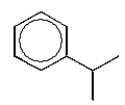
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
12
The name of the following molecule is: 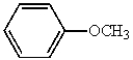
A) toluene
B) ethylbenzene
C) cumene
D) styrene
E) anisole

A) toluene
B) ethylbenzene
C) cumene
D) styrene
E) anisole

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
13
The structural formula for p-chlorobenzoic acid is:
A)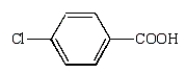
B)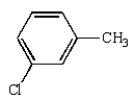
C)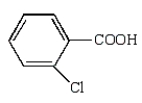
D)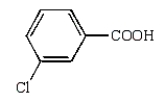
E)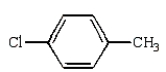
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
14
What is the name of the following molecule?
PhCH2CH2CH=CH2
A) styrene
B) 4-phenyl-1-butene
C) 1-phenyl-3-butene
D) 3-benzyl-1-propene
E) allylbenzene
PhCH2CH2CH=CH2
A) styrene
B) 4-phenyl-1-butene
C) 1-phenyl-3-butene
D) 3-benzyl-1-propene
E) allylbenzene

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
15
The structure of chlorobenzene is correctly represented by:
A)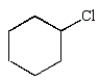
B)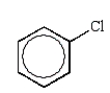
C)
D)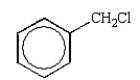
E)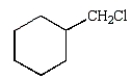
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
16
What is the correct name for the following molecule? 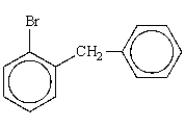
A) o-bromobenzyl
B) biphenyl bromide
C) 2-bromodiphenylpropane
D) bromobenzylbenzene
E) o-benzylbromobenzene

A) o-bromobenzyl
B) biphenyl bromide
C) 2-bromodiphenylpropane
D) bromobenzylbenzene
E) o-benzylbromobenzene

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
17
The correct name for 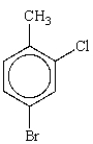 is
is
A) 2-chloro-4-bromotoluene.
B) o-chloro-p-bromotoluene.
C) 1-bromo-3-chloro-4-methylbenzene.
D) 4-bromo-2-chlorotoluene.
E) m-chlorobromotoluene.
 is
isA) 2-chloro-4-bromotoluene.
B) o-chloro-p-bromotoluene.
C) 1-bromo-3-chloro-4-methylbenzene.
D) 4-bromo-2-chlorotoluene.
E) m-chlorobromotoluene.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
18
Which position would be para to X? 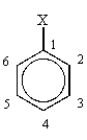
A) 1
B) 2
C) 3
D) 4
E) 6

A) 1
B) 2
C) 3
D) 4
E) 6

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
19
In the mechanism for the nitration of benzene, what is the function of H2SO4?
A) to act solely as a solvent
B) to donate a proton to HNO3
C) to accept a proton from HNO3
D) to generate heat for reaction to occur
E) to protonate the benzene ring
A) to act solely as a solvent
B) to donate a proton to HNO3
C) to accept a proton from HNO3
D) to generate heat for reaction to occur
E) to protonate the benzene ring

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
20
How many dinitrobenzoic acids are possible?
A) 4
B) 5
C) 6
D) 7
E) 8
A) 4
B) 5
C) 6
D) 7
E) 8

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following groups are ortho, para-directing?
A) -CO2CH3
B) -CONH2
C) -SO3H
D)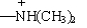
E) -SCH3
A) -CO2CH3
B) -CONH2
C) -SO3H
D)
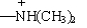
E) -SCH3

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
22
The predominant intermediate in electrophilic nitration of toluene is:
A)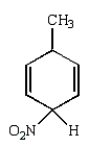
B)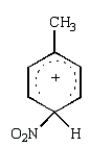
C)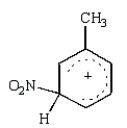
D)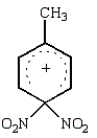
E)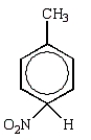
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
23
The predominant product from sequential nitration and bromination of benzenesulfonic acid is:
A)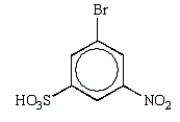
B)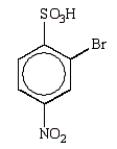
C)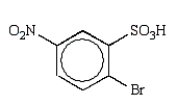
D)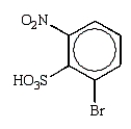
E)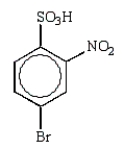
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
24
If p-nitrophenol is treated with chlorine in the presence of AlCl3, the only trisubstituted product observed is:
A) 2-chloro-4-nitrophenol
B) 3-chloro-4-nitrophenol
C) 3-chloro-5-nitrophenol
D) 4-chloro-2-nitrophenol
E) 4-chloro-3-nitrophenol
A) 2-chloro-4-nitrophenol
B) 3-chloro-4-nitrophenol
C) 3-chloro-5-nitrophenol
D) 4-chloro-2-nitrophenol
E) 4-chloro-3-nitrophenol

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
25
What is the product of the following reaction? 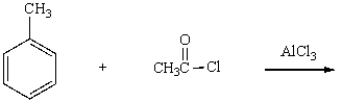
A)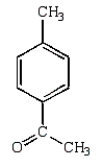
B)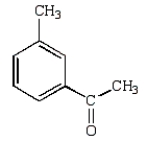
C)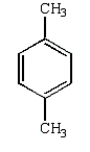
D)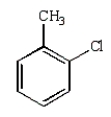
E)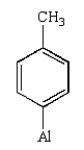

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
26
The relative rates of nitration of the following are: 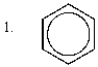
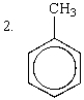
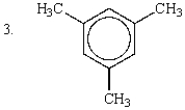
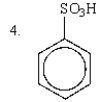
A) 1 > 2 > 3 > 4
B) 4 > 2 > 1 > 3
C) 2 > 1 > 4 > 3
D) 3 > 4 > 2 > 1
E) 3 > 2 > 1 > 4




A) 1 > 2 > 3 > 4
B) 4 > 2 > 1 > 3
C) 2 > 1 > 4 > 3
D) 3 > 4 > 2 > 1
E) 3 > 2 > 1 > 4

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
27
The only group among the following that is m-directing is:
A)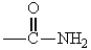
B)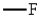
C)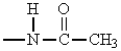
D)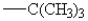
E)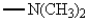
A)
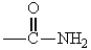
B)
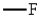
C)
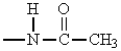
D)
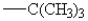
E)
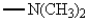

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
28
The expected product from the following reaction is: 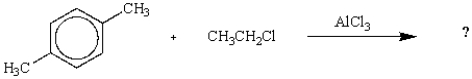
A)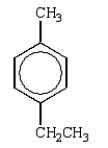
B)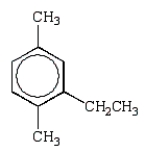
C)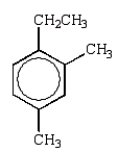
D)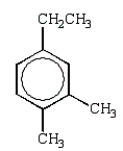
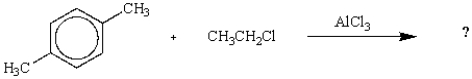
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
29
In electrophilic aromatic substitution reactions, which of the following molecules are considered to be less reactive than benzene?
A)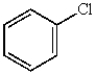
B)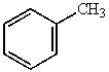
C)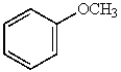
D)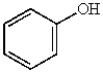
E)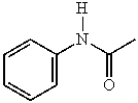
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
30
The predominant intermediate in the nitration of benzenesulfonic acid is:
A)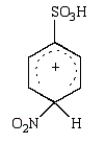
B)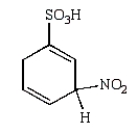
C)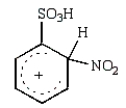
D)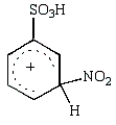
E)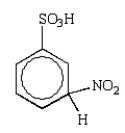
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following molecules is the most reactive toward electrophilic aromatic substitution?
A)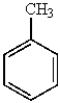
B)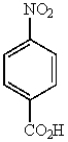
C)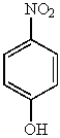
D)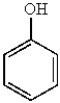
E)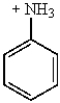
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
32
Which electrophile is used to make acetophenone from benzene?
A)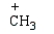
B)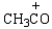
C)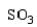
D)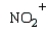
E)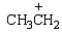
A)
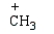
B)
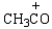
C)
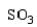
D)
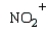
E)
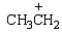

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
33
What is the name of the major product from the following sequence of reactions? 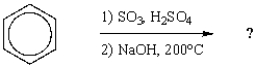
A) aniline
B) anisole
C) benzoic acid
D) phenol
E) toluene
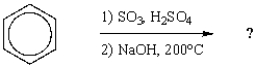
A) aniline
B) anisole
C) benzoic acid
D) phenol
E) toluene

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
34
What is the best sequence of reactions to synthesize m-nitrophenol?
A)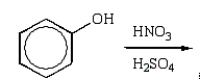
B)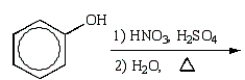
C)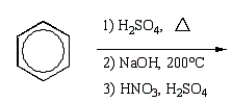
D)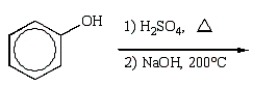
E)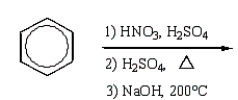
A)

B)
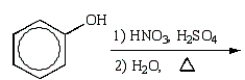
C)

D)
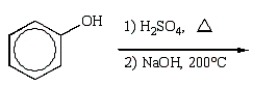
E)
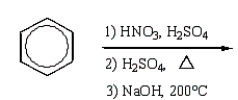

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
35
The name of the product of the following reaction is: 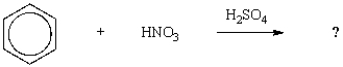
A) benzenesulfonic acid
B) aniline
C) benzoic acid
D) nitrobenzene
E) anisole
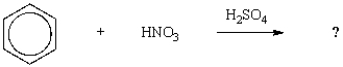
A) benzenesulfonic acid
B) aniline
C) benzoic acid
D) nitrobenzene
E) anisole

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
36
The predominant product from the sequential bromination and nitration of benzene is:
A)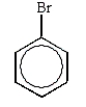
B)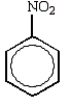
C)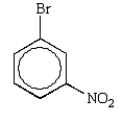
D)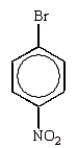
E)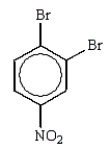
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
37
Among the following groups, which ones are o,p-directing? 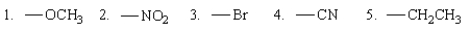
A) 1, 3, and 5
B) 1 and 5
C) 2 and 4
D) 2, 3, and 4
E) 1 and 3
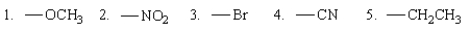
A) 1, 3, and 5
B) 1 and 5
C) 2 and 4
D) 2, 3, and 4
E) 1 and 3

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following groups is a meta director?
A) -Cl
B) -COOH
C) -OCH3
D) -OH
E) -NH2
A) -Cl
B) -COOH
C) -OCH3
D) -OH
E) -NH2

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
39
Which group is both ortho-para directing and ring-deactivating?
A) -Cl
B) -CH3
C) -NO2
D) -CHO
E) -OCH3
A) -Cl
B) -CH3
C) -NO2
D) -CHO
E) -OCH3

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
40
What is the final product of the following reaction sequence? 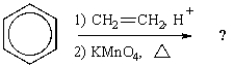
A)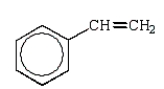
B)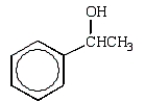
C)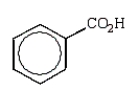
D)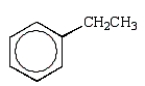
E)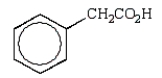
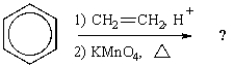
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
41
Which is the best reaction sequence to synthesize m-bromobenzenesulfonic acid from benzene? 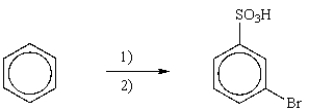
A) 1) Br2, AlBr3, 2) H2SO4, SO3
B) 1) H2SO4, SO3 2) Br2, AlBr3
C) 1) ethene, HF, 2) Br2, AlBr3
D) 1) CH3Cl, AlCl3, 2) Br2, AlBr3
E) 1) Br2, AlBr3, 2) CH3COCl, AlCl3

A) 1) Br2, AlBr3, 2) H2SO4, SO3
B) 1) H2SO4, SO3 2) Br2, AlBr3
C) 1) ethene, HF, 2) Br2, AlBr3
D) 1) CH3Cl, AlCl3, 2) Br2, AlBr3
E) 1) Br2, AlBr3, 2) CH3COCl, AlCl3

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
42
The number of possible mononitration products of anthracene is: 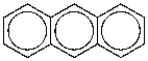
A) 1
B) 2
C) 3
D) 4
E) 5

A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
43
The polycyclic aromatic hydrocarbon benzo[a]pyrene is a known carcinogen found in soot and tobacco smoke.What is its structure?
A)![<strong>The polycyclic aromatic hydrocarbon benzo[a]pyrene is a known carcinogen found in soot and tobacco smoke.What is its structure?</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB7360/11eadbaf_29f8_0053_a3bf_238bd18b2f3f_TB7360_00.jpg)
B)![<strong>The polycyclic aromatic hydrocarbon benzo[a]pyrene is a known carcinogen found in soot and tobacco smoke.What is its structure?</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB7360/11eadbaf_29f8_0054_a3bf_17212b6c887c_TB7360_00.jpg)
C)![<strong>The polycyclic aromatic hydrocarbon benzo[a]pyrene is a known carcinogen found in soot and tobacco smoke.What is its structure?</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB7360/11eadbaf_29f8_0055_a3bf_a7d9ae81e6d8_TB7360_00.jpg)
D)![<strong>The polycyclic aromatic hydrocarbon benzo[a]pyrene is a known carcinogen found in soot and tobacco smoke.What is its structure?</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB7360/11eadbaf_29f8_2766_a3bf_ad888d178b38_TB7360_00.jpg)
E)![<strong>The polycyclic aromatic hydrocarbon benzo[a]pyrene is a known carcinogen found in soot and tobacco smoke.What is its structure?</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB7360/11eadbaf_29f8_2767_a3bf_e1c38e141446_TB7360_00.jpg)
A)
![<strong>The polycyclic aromatic hydrocarbon benzo[a]pyrene is a known carcinogen found in soot and tobacco smoke.What is its structure?</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB7360/11eadbaf_29f8_0053_a3bf_238bd18b2f3f_TB7360_00.jpg)
B)
![<strong>The polycyclic aromatic hydrocarbon benzo[a]pyrene is a known carcinogen found in soot and tobacco smoke.What is its structure?</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB7360/11eadbaf_29f8_0054_a3bf_17212b6c887c_TB7360_00.jpg)
C)
![<strong>The polycyclic aromatic hydrocarbon benzo[a]pyrene is a known carcinogen found in soot and tobacco smoke.What is its structure?</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB7360/11eadbaf_29f8_0055_a3bf_a7d9ae81e6d8_TB7360_00.jpg)
D)
![<strong>The polycyclic aromatic hydrocarbon benzo[a]pyrene is a known carcinogen found in soot and tobacco smoke.What is its structure?</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB7360/11eadbaf_29f8_2766_a3bf_ad888d178b38_TB7360_00.jpg)
E)
![<strong>The polycyclic aromatic hydrocarbon benzo[a]pyrene is a known carcinogen found in soot and tobacco smoke.What is its structure?</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB7360/11eadbaf_29f8_2767_a3bf_e1c38e141446_TB7360_00.jpg)

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
44
Which is the best reaction sequence to synthesize p-bromobenzenesulfonic acid from benzene? 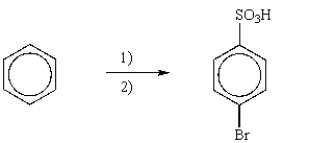
A) 1) Br2, AlBr3, 2) H2SO4, SO3
B) 1) H2SO4, SO3, 2) Br2, AlBr3
C) 1) CH3Cl, AlCl3, 2) Br2, AlBr3
D) 1) Br2, AlBr3, 2) CH3COCl, AlCl3
E) 1) HBr, ethane, 2) H2SO4, SO3

A) 1) Br2, AlBr3, 2) H2SO4, SO3
B) 1) H2SO4, SO3, 2) Br2, AlBr3
C) 1) CH3Cl, AlCl3, 2) Br2, AlBr3
D) 1) Br2, AlBr3, 2) CH3COCl, AlCl3
E) 1) HBr, ethane, 2) H2SO4, SO3

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
45
A common polycyclic aromatic hydrocarbon is named naphthalene.What is the structure of naphthalene?
A)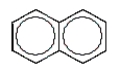
B)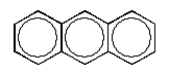
C)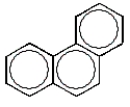
D)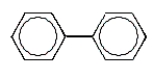
E)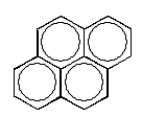
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
46
Which is the best sequence of reagents to use in synthesizing 2-chloro-4-nitrotoluene from benzene:
A) Cl2, FeCl3; then CH3Cl, AlCl3; then HNO3, H2SO4
B) CH3Cl, AlCl3; then Cl2, FeCl3; then HNO3, H2SO4
C) CH3Cl, AlCl3; then HNO3, H2SO4; then Cl2, FeCl3
D) SO3, H2SO4; then HNO3, H2SO4; then Cl2, FeCl3
E) HNO3, H2SO4; then Cl2, FeCl3; then CH3Cl, AlCl3
A) Cl2, FeCl3; then CH3Cl, AlCl3; then HNO3, H2SO4
B) CH3Cl, AlCl3; then Cl2, FeCl3; then HNO3, H2SO4
C) CH3Cl, AlCl3; then HNO3, H2SO4; then Cl2, FeCl3
D) SO3, H2SO4; then HNO3, H2SO4; then Cl2, FeCl3
E) HNO3, H2SO4; then Cl2, FeCl3; then CH3Cl, AlCl3

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck



