Deck 2: Alkanes and Cycloalkanes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/43
Play
Full screen (f)
Deck 2: Alkanes and Cycloalkanes
1
The IUPAC name for the following molecule is: 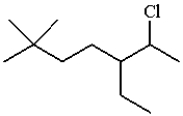
A) 2-chloro-3-ethyl-6,6-dimethylheptane
B) 6-chloro-5-ethyl-2,2-dimethylheptane
C) 6-chloro-2,2-dimethyl-5-ethylheptane
D) 2,2-dimethyl-5-chloroethylheptane
E) 6-chloro-5-ethyl-2-dimethylheptane

A) 2-chloro-3-ethyl-6,6-dimethylheptane
B) 6-chloro-5-ethyl-2,2-dimethylheptane
C) 6-chloro-2,2-dimethyl-5-ethylheptane
D) 2,2-dimethyl-5-chloroethylheptane
E) 6-chloro-5-ethyl-2-dimethylheptane
6-chloro-5-ethyl-2,2-dimethylheptane
2
The name of the alkyl group that contains three carbons is:
A) methyl
B) ethyl
C) propyl
D) isopropyl
E) none of these
A) methyl
B) ethyl
C) propyl
D) isopropyl
E) none of these
propyl
3
What statement does NOT apply to the boiling points of alkanes?
A) The boiling point increases as the length of the carbon chain increases.
B) Straight chain alkanes have a higher boiling point than their branched isomers.
C) Because they are nonpolar, alkanes have lower boiling points than other organic compounds of similar molar mass.
D) The boiling points are affected by Van der Waals attractions.
E) The boiling points are influenced by hydrogen bonding.
A) The boiling point increases as the length of the carbon chain increases.
B) Straight chain alkanes have a higher boiling point than their branched isomers.
C) Because they are nonpolar, alkanes have lower boiling points than other organic compounds of similar molar mass.
D) The boiling points are affected by Van der Waals attractions.
E) The boiling points are influenced by hydrogen bonding.
The boiling points are influenced by hydrogen bonding.
4
What is the molecular formula of an alkane that has fourteen carbon atoms?
A) C14H28
B) C14H30
C) C14H32
D) C14H34
E) C14H26
A) C14H28
B) C14H30
C) C14H32
D) C14H34
E) C14H26

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
5
What is the molecular formula of a cycloalkane that has six carbon atoms?
A) C6H12
B) C6H14
C) C6H16
D) C6H10
E) C6H7
A) C6H12
B) C6H14
C) C6H16
D) C6H10
E) C6H7

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
6
The structural formula for 2,2,3-trimethylhexane is
A)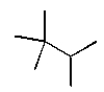
B)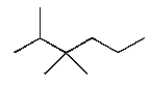
C)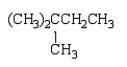
D)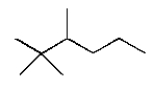
E)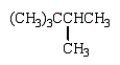
A)

B)

C)
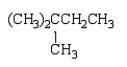
D)

E)
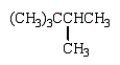

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
7
What is a correct name for the following molecule? 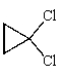
A) 2,2-dichlorocyclopropane
B) 1,1-dichlorocyclopentane
C) 1,1-dichloropropane
D) trans-1,1-dichlorocyclopropane
E) 1,1-dichlorocyclopropane

A) 2,2-dichlorocyclopropane
B) 1,1-dichlorocyclopentane
C) 1,1-dichloropropane
D) trans-1,1-dichlorocyclopropane
E) 1,1-dichlorocyclopropane

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
8
What is the correct name for the following cycloalkane? 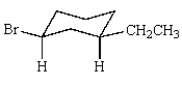
A) bromoethylcyclohexane
B) trans-1-ethyl-3-bromocyclohexane
C) cis-3-bromo-1-ethylhexane
D) 1-bromo-3-ethylcyclohexane
E) cis-1-bromo-3-ethylcyclohexane

A) bromoethylcyclohexane
B) trans-1-ethyl-3-bromocyclohexane
C) cis-3-bromo-1-ethylhexane
D) 1-bromo-3-ethylcyclohexane
E) cis-1-bromo-3-ethylcyclohexane

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
9
The IUPAC name for the following molecule is: 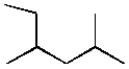
A) 2-ethyl-4-methylpentane
B) 4-methyl-2-methylpentane
C) 2,4-dimethylhexane
D) 1-isopropyl-2-methylbutane
E) 2,4-methylhexane

A) 2-ethyl-4-methylpentane
B) 4-methyl-2-methylpentane
C) 2,4-dimethylhexane
D) 1-isopropyl-2-methylbutane
E) 2,4-methylhexane

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following would exhibit hydrogen bonding?
A) CH3Cl
B) CH3OH
C) CH4
D) CH2Cl2
E) CH3CH3
A) CH3Cl
B) CH3OH
C) CH4
D) CH2Cl2
E) CH3CH3

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following alkanes would have the highest boiling point?
A) pentane
B) 2-methylbutane
C) 2,2-dimethylpropane
D) hexane
E) 2-methylpentane
A) pentane
B) 2-methylbutane
C) 2,2-dimethylpropane
D) hexane
E) 2-methylpentane

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
12
What is the common name for the following molecule? 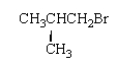
A) isobutyl bromide
B) tert-butyl bromide
C) butyl bromide
D) sec-butyl bromide
E) bromo-sec-butane

A) isobutyl bromide
B) tert-butyl bromide
C) butyl bromide
D) sec-butyl bromide
E) bromo-sec-butane

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
13
The name of the alkyl group below is: 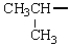
A) ethyl
B) propyl
C) isopropyl
D) butyl
E) isobutyl
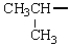
A) ethyl
B) propyl
C) isopropyl
D) butyl
E) isobutyl

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
14
What is the name of the alkane that has three carbon atoms?
A) methane
B) ethane
C) propane
D) butane
E) isobutane
A) methane
B) ethane
C) propane
D) butane
E) isobutane

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
15
The correct IUPAC name for (CH3)2CHCH(CH3)(CH2)3CH(CH3)2 is
A) diisopropylpentane.
B) 1,1,2,6,6-pentamethylhexane.
C) 2,5-diisopropylpentane.
D) 2,3,7-trimethyloctane.
E) 1,4-diisopropylpentane.
A) diisopropylpentane.
B) 1,1,2,6,6-pentamethylhexane.
C) 2,5-diisopropylpentane.
D) 2,3,7-trimethyloctane.
E) 1,4-diisopropylpentane.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
16
The correct IUPAC name for 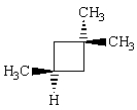 is:
is:
A) 1,3,3-trimethylcyclobutane.
B) cis-1,3,3-trimethylcyclobutane.
C) trans-1,3,3-trimethylcyclobutane.
D) 1,1,3-trimethylcyclobutane.
E) 2,2,4-trimethylcyclobutane.
 is:
is:A) 1,3,3-trimethylcyclobutane.
B) cis-1,3,3-trimethylcyclobutane.
C) trans-1,3,3-trimethylcyclobutane.
D) 1,1,3-trimethylcyclobutane.
E) 2,2,4-trimethylcyclobutane.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following structures is 2-methylpentane?
A)
B)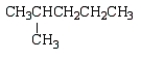
C)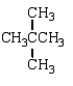
D)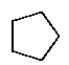
E)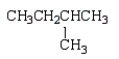
A)

B)
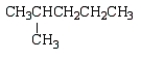
C)

D)

E)
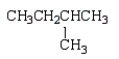

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
18
What is the IUPAC name for the following compound? 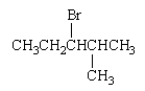
A) isohexyl bromide
B) 3-bromo-4-methylpentane
C) 1-bromopropylpropane
D) 3-bromo-2-methylpentane
E) 2-methyl-3-bromopentane

A) isohexyl bromide
B) 3-bromo-4-methylpentane
C) 1-bromopropylpropane
D) 3-bromo-2-methylpentane
E) 2-methyl-3-bromopentane

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
19
Trans-1-bromo-3-methylcyclobutane is represented by which structure below?
A)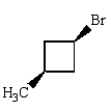
B)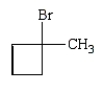
C)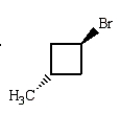
D)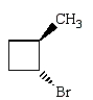
E)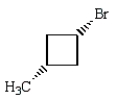
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
20
The correct IUPAC name for the following molecule is: 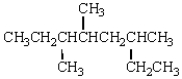
A) 6-ethyl-3,4,-dimethylheptane
B) 2-ethyl-4,5-dimethylheptane
C) 3,4,6-trimethyloctane
D) 3,5,6-trimethyloctane
E) none of these

A) 6-ethyl-3,4,-dimethylheptane
B) 2-ethyl-4,5-dimethylheptane
C) 3,4,6-trimethyloctane
D) 3,5,6-trimethyloctane
E) none of these

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
21
The preferred conformation of butane is given by which of the following Newman projection formulas?
A)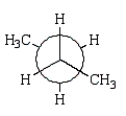
B)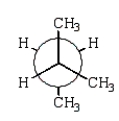
C)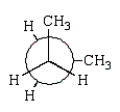
D)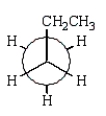
E)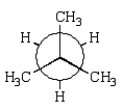
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
22
The boiling points of normal alkanes
A) rise as the length of the carbon chain increases.
B) rise as the length of the carbon chain decreases.
C) are higher than the boiling points of branched alkanes with the same molecular formula.
D) a and c
E) b and c
A) rise as the length of the carbon chain increases.
B) rise as the length of the carbon chain decreases.
C) are higher than the boiling points of branched alkanes with the same molecular formula.
D) a and c
E) b and c

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
23
The compounds represented by the structures below are: 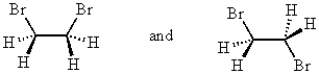
A) structural isomers.
B) different compounds.
C) cis-trans isomers.
D) conformers.
E) constitutional isomers.
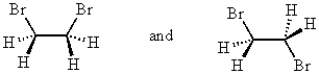
A) structural isomers.
B) different compounds.
C) cis-trans isomers.
D) conformers.
E) constitutional isomers.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
24
Cycloalkanes with __________ or more carbons in the ring are nonplanar.
A) 2
B) 3
C) 4
D) 5
E) 6
A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
25
The compounds represented by the structures below are: 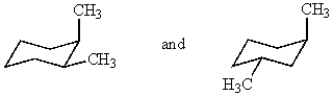
A) constitutional isomers.
B) identical.
C) cis-trans isomers.
D) conformers.
E) different compounds (not isomers)..
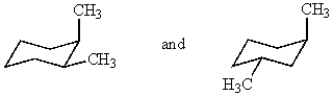
A) constitutional isomers.
B) identical.
C) cis-trans isomers.
D) conformers.
E) different compounds (not isomers)..

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
26
How many isomeric dichloro products can be obtained from the chlorination of cyclopropane?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
27
For the most stable conformation of cis-1,3-dimethylcyclohexane:
A) both methyls will occupy the axial position
B) both methyls will occupy the equatorial position
C) one methyl will occupy the axial position and the other an equatorial position
D) more than one answer is correct
A) both methyls will occupy the axial position
B) both methyls will occupy the equatorial position
C) one methyl will occupy the axial position and the other an equatorial position
D) more than one answer is correct

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
28
The preferred conformation of cis-3-tert-butyl-1-methylcyclohexane is the one in which:
A) the t-butyl group is axial and the methyl group is equatorial
B) both groups are axial
C) both groups are equatorial
D) the methyl group is axial and the t-butyl group is equatorial
E) molecule exists in a boat conformation
A) the t-butyl group is axial and the methyl group is equatorial
B) both groups are axial
C) both groups are equatorial
D) the methyl group is axial and the t-butyl group is equatorial
E) molecule exists in a boat conformation

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following pairs are examples of conformational isomerism?
A) chair and boat forms of cyclohexane
B) 1-iodopropane and 2-iodopropane
C) sec-butyl chloride and butyl iodide
D) cis and trans-1,2-dimethylcyclohexane
E) all of these
A) chair and boat forms of cyclohexane
B) 1-iodopropane and 2-iodopropane
C) sec-butyl chloride and butyl iodide
D) cis and trans-1,2-dimethylcyclohexane
E) all of these

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
30
How many monobromo products can be obtained from the bromination of cyclopentane?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
31
The least stable conformation of butane is given by which of the following Newman projections?
A)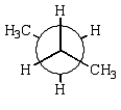
B)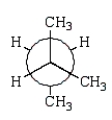
C)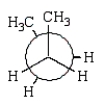
D)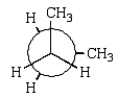
E)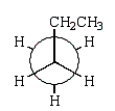
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
32
The most stable conformation of propane is:
A) staggered
B) chair
C) planar
D) eclipsed
E) boat
A) staggered
B) chair
C) planar
D) eclipsed
E) boat

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
33
The compounds represented by the structures below are: 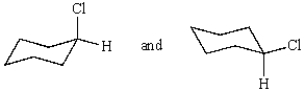
A) structural isomers.
B) different compounds.
C) cis-trans isomers.
D) conformers.
E) constitutional isomers.
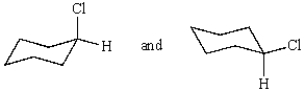
A) structural isomers.
B) different compounds.
C) cis-trans isomers.
D) conformers.
E) constitutional isomers.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
34
The least stable conformation of propane is:
A) staggered
B) chair
C) planar
D) eclipsed
E) boat
A) staggered
B) chair
C) planar
D) eclipsed
E) boat

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
35
Consider this chair conformation: 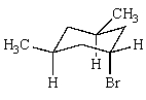 When the ring flips,
When the ring flips,
A) the bromine becomes axial and the methyls become equatorial.
B) all three substituents become equatorial.
C) the bromine becomes equatorial and the methyls become axial.
D) the ring opens up.
E) one methyl becomes axial, one becomes equatorial, and the bromine becomes equatorial.
 When the ring flips,
When the ring flips,A) the bromine becomes axial and the methyls become equatorial.
B) all three substituents become equatorial.
C) the bromine becomes equatorial and the methyls become axial.
D) the ring opens up.
E) one methyl becomes axial, one becomes equatorial, and the bromine becomes equatorial.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
36
In the chlorination of methane, the propagation steps involve forming:
A) H radicals
B) methyl radicals
C) chlorine radicals
D) a, b, and c
E) b and c
A) H radicals
B) methyl radicals
C) chlorine radicals
D) a, b, and c
E) b and c

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
37
The bond angle of a normal, tetrahedral, sp3 hybridized carbon is 109.5°.What is the C-C-C bond angle of cyclobutane?
A) 60°
B) 90°
C) 109.5°
D) 120°
E) 180°
A) 60°
B) 90°
C) 109.5°
D) 120°
E) 180°

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
38
Consider this chair conformation: 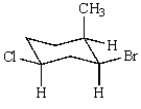
A) The methyl and bromine are cis and the chlorine and bromine are cis.
B) The methyl and bromine are trans and the chlorine and bromine are cis.
C) The methyl and chlorine are trans and the methyl and bromine are cis.
D) The methyl and chlorine are trans and the methyl and bromine are trans.
E) The methyl and chlorine are trans and the bromine and chlorine are cis.

A) The methyl and bromine are cis and the chlorine and bromine are cis.
B) The methyl and bromine are trans and the chlorine and bromine are cis.
C) The methyl and chlorine are trans and the methyl and bromine are cis.
D) The methyl and chlorine are trans and the methyl and bromine are trans.
E) The methyl and chlorine are trans and the bromine and chlorine are cis.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
39
The compounds represented by the structures below are: 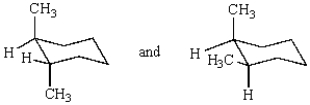
A) structural isomers.
B) identical.
C) cis-trans isomers.
D) conformers.
E) constitutional isomers.
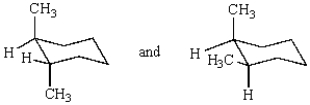
A) structural isomers.
B) identical.
C) cis-trans isomers.
D) conformers.
E) constitutional isomers.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
40
Which cycloalkane has the highest boiling point?
A) cyclopropane
B) cyclobutane
C) cyclopentane
D) cyclohexane
E) cyclooctane
A) cyclopropane
B) cyclobutane
C) cyclopentane
D) cyclohexane
E) cyclooctane

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
41
The number of possible dichlorination products of propane is
A) 2
B) 3
C) 4
D) 5
E) 6
A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
42
The number of possible dibromination products of 2-methylpropane is
A) 2
B) 3
C) 4
D) 5
E) 6
A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
43
The number of possible monobromination products, including cis-trans isomers, of methylcyclopentane is
A) 2
B) 3
C) 4
D) 5
E) 6
A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck



