Deck 6: Chemical Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/47
Play
Full screen (f)
Deck 6: Chemical Reactions
1
Which equation would be described as a single displacement reaction?
A) 2 CO + O2 2 CO2
B) CuCO3 CuO + CO2
C) 2 AgNO3 + Na2CrO4 Ag2CrO4 + 2 NaNO3
D) Zn + Cu(NO3)2 Zn(NO3)2 + Cu
A) 2 CO + O2 2 CO2
B) CuCO3 CuO + CO2
C) 2 AgNO3 + Na2CrO4 Ag2CrO4 + 2 NaNO3
D) Zn + Cu(NO3)2 Zn(NO3)2 + Cu
Zn + Cu(NO3)2 Zn(NO3)2 + Cu
2
Which equation would be described as a synthesis (combination) reaction?
A) 2 CO + O2 2 CO2
B) CuCO3 CuO + CO2
C) 2 AgNO3 + Na2CrO4 Ag2CrO4 + 2 NaNO3
D) Zn + Cu(NO3)2 Zn(NO3)2 + Cu
A) 2 CO + O2 2 CO2
B) CuCO3 CuO + CO2
C) 2 AgNO3 + Na2CrO4 Ag2CrO4 + 2 NaNO3
D) Zn + Cu(NO3)2 Zn(NO3)2 + Cu
2 CO + O2 2 CO2
3
When heated, iron and sulfur combine to form a new compound, iron(II) sulfide. In this reaction:
A) both Fe and S are oxidized.
B) both Fe and S are reduced.
C) Fe is oxidized and S is reduced.
D) S is oxidized and Fe is reduced.
A) both Fe and S are oxidized.
B) both Fe and S are reduced.
C) Fe is oxidized and S is reduced.
D) S is oxidized and Fe is reduced.
Fe is oxidized and S is reduced.
4
When magnesium and chlorine react, what is the formula of the resulting compound?
A) MgCl
B) Mg2Cl
C) MgCl2
D) Mg2Cl2
A) MgCl
B) Mg2Cl
C) MgCl2
D) Mg2Cl2

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
5
What is the coefficient of hydrogen when the following equation is balanced? __ Na + __ H2O __ NaOH + __ H2
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
6
What is the coefficient of cobalt when the following equation is balanced?
__ Co + __ O2 __ Co2O3
A) 1
B) 2
C) 3
D) 4
__ Co + __ O2 __ Co2O3
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
7
Which balanced equation BEST describes this reaction? 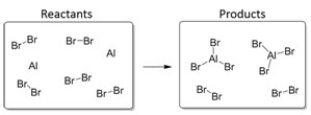
A) Al2 + 6 Br Al2Br6 + Br4
B) 2 Al + 5 Br2 2 AlBr3 + 2 Br2
C) 2 Al + 3 Br2 2 AlBr3
D) 2 Al + 6 Br 2 AlBr3

A) Al2 + 6 Br Al2Br6 + Br4
B) 2 Al + 5 Br2 2 AlBr3 + 2 Br2
C) 2 Al + 3 Br2 2 AlBr3
D) 2 Al + 6 Br 2 AlBr3

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
8
When calcium carbonate is heated, it forms two new compounds, calcium oxide and carbon dioxide. This is an example of a _____ reaction.
A) decomposition
B) synthesis
C) single displacement
D) double displacement
A) decomposition
B) synthesis
C) single displacement
D) double displacement

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
9
Which choice BEST represents a balanced equation for the reaction of nitrogen gas and oxygen gas to form nitrogen monoxide?
A) N2 + O2 2 NO
B) N + O NO
C) N2 + 2 O 2 NO
D) 2 N + O2 2 NO
A) N2 + O2 2 NO
B) N + O NO
C) N2 + 2 O 2 NO
D) 2 N + O2 2 NO

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
10
When aluminum and sulfur react, what is the formula of the resulting compound?
A) AlS
B) Al3S2
C) Al2S3
D) AlS3
A) AlS
B) Al3S2
C) Al2S3
D) AlS3

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
11
Which choice BEST represents a balanced equation for the reaction of nitrogen gas with hydrogen gas to produce ammonia?
A) N2 + 3 H2 2 NH3
B) 2 N + 3 H NH3
C) N2 + 6 H 2 NH3
D) N + 3 H NH3
A) N2 + 3 H2 2 NH3
B) 2 N + 3 H NH3
C) N2 + 6 H 2 NH3
D) N + 3 H NH3

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
12
When solutions of potassium iodide and lead(II) nitrate are mixed, the result is potassium nitrate and lead(II) iodide. This is an example of a _____ reaction.
A) decomposition
B) synthesis
C) single displacement
D) double displacement
A) decomposition
B) synthesis
C) single displacement
D) double displacement

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
13
What is the coefficient of potassium chloride when the following equation is balanced?
__ KClO3 __ KCl + __ O2
A) 1
B) 2
C) 3
D) 4
__ KClO3 __ KCl + __ O2
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
14
Which equation would be described as a double displacement reaction?
A) 2 CO + O2 2 CO2
B) CuCO3 CuO + CO2
C) 2 AgNO3 + Na2CrO4 Ag2CrO4 + 2 NaNO3
D) Zn + Cu(NO3)2 Zn(NO3)2 + Cu
A) 2 CO + O2 2 CO2
B) CuCO3 CuO + CO2
C) 2 AgNO3 + Na2CrO4 Ag2CrO4 + 2 NaNO3
D) Zn + Cu(NO3)2 Zn(NO3)2 + Cu

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
15
When magnesium burns in oxygen, the result is magnesium oxide. This is an example of a _____ reaction.
A) decomposition
B) synthesis
C) single displacement
D) double displacement
A) decomposition
B) synthesis
C) single displacement
D) double displacement

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
16
When aluminum is placed in a copper(II) chloride solution, the result is copper and aluminum chloride. This is an example of a _____ reaction.
A) decomposition
B) synthesis
C) single displacement
D) double displacement
A) decomposition
B) synthesis
C) single displacement
D) double displacement

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
17
When potassium and fluorine react, what is the formula of the resulting compound?
A) KF
B) K2F
C) KF2
D) K2F2
A) KF
B) K2F
C) KF2
D) K2F2

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
18
Which choice BEST represents a balanced equation for the reaction of nitrogen gas and chlorine gas to form nitrogen trichloride?
A) N2 + 3 Cl2 2 NCl3
B) 2 N + 3 Cl NCl3
C) N2 + 6 Cl 2 NCl3
D) N + 3 Cl NCl3
A) N2 + 3 Cl2 2 NCl3
B) 2 N + 3 Cl NCl3
C) N2 + 6 Cl 2 NCl3
D) N + 3 Cl NCl3

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
19
Which equation would be described as a decomposition reaction?
A) 2 CO + O2 2 CO2
B) CuCO3 CuO + CO2
C) 2 AgNO3 + Na2CrO4 Ag2CrO4 + 2 NaNO3
D) Zn + Cu(NO3)2 Zn(NO3)2 + Cu
A) 2 CO + O2 2 CO2
B) CuCO3 CuO + CO2
C) 2 AgNO3 + Na2CrO4 Ag2CrO4 + 2 NaNO3
D) Zn + Cu(NO3)2 Zn(NO3)2 + Cu

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
20
What is the coefficient of carbon dioxide when the following equation is balanced?
__ Fe2(CO3)3 __ Fe2O3 + __ CO2
A) 1
B) 2
C) 3
D) 4
__ Fe2(CO3)3 __ Fe2O3 + __ CO2
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
21
According to the solubility rules, which compound is SOLUBLE in water?
A) CaCO3
B) PbCl2
C) Cu(NO3)2
D) CaSO4
A) CaCO3
B) PbCl2
C) Cu(NO3)2
D) CaSO4

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
22
The equation that shows the applicable dissociation of the reactants and products is called the _____ equation.
A) net ionic
B) ionic
C) molecular
D) algebraic
A) net ionic
B) ionic
C) molecular
D) algebraic

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
23
Based on the solubility rules, what precipitate results from combining aqueous potassium bromide and aqueous lead(II) nitrate?
A) PbBr2
B) KBr
C) PbK
D) KNO3
A) PbBr2
B) KBr
C) PbK
D) KNO3

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
24
Consider the balanced equation representing the complete combustion of ethylene, C2H4. What is the coefficient of the oxygen?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
25
Based on the solubility rules, what precipitate results from combining aqueous sodium iodide and aqueous silver nitrate?
A) AgI
B) NaNO3
C) AgNa
D) AgNO3
A) AgI
B) NaNO3
C) AgNa
D) AgNO3

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
26
What products form from the neutralization reaction of HCl with sodium hydroxide?
A) NaClO2 and H2
B) H2O and NaCl
C) NaH and ClO2
D) O2 and NaHCl
A) NaClO2 and H2
B) H2O and NaCl
C) NaH and ClO2
D) O2 and NaHCl

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
27
The equation that shows all the reactant and product formulas is called the _____ equation.
A) net ionic
B) ionic
C) molecular
D) algebraic
A) net ionic
B) ionic
C) molecular
D) algebraic

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
28
What are the products of the complete combustion of a hydrocarbon?
A) CO and H2O
B) CO and H2
C) CO2 and H2
D) CO2 and H2O
A) CO and H2O
B) CO and H2
C) CO2 and H2
D) CO2 and H2O

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
29
When heated, magnesium and nitrogen combine to form a new compound, magnesium nitride. In this reaction:
A) both Mg and N are oxidized.
B) both Mg and N are reduced.
C) N is oxidized and Mg is reduced.
D) Mg is oxidized and N is reduced.
A) both Mg and N are oxidized.
B) both Mg and N are reduced.
C) N is oxidized and Mg is reduced.
D) Mg is oxidized and N is reduced.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
30
According to the solubility rules, which compound is INSOLUBLE in water?
A) KBr
B) Na2CO3
C) Cu(OH)2
D) AgClO4
A) KBr
B) Na2CO3
C) Cu(OH)2
D) AgClO4

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
31
What products form from the neutralization reaction of HBr with lithium hydroxide?
A) LiBrO2 and H2
B) H2O and LiBr
C) LiH and BrO2
D) O2 and LiHBr
A) LiBrO2 and H2
B) H2O and LiBr
C) LiH and BrO2
D) O2 and LiHBr

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
32
Based on the solubility rules, what precipitate results from combining aqueous ammonium sulfate and aqueous barium nitrate?
A) ammonium nitrate
B) barium sulfate
C) barium ammonium
D) barium nitrate
A) ammonium nitrate
B) barium sulfate
C) barium ammonium
D) barium nitrate

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
33
Consider the balanced equation representing the complete combustion of ethylene, C2H4. What is the coefficient of the ethylene?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
34
What products form from the neutralization reaction of HI with potassium hydroxide?
A) KIO2 and H2
B) H2O and KI
C) KH and IO2
D) O2 and KHI
A) KIO2 and H2
B) H2O and KI
C) KH and IO2
D) O2 and KHI

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
35
Which equation BEST represents the combustion of propane, C3H8?
A) C3H8 + O2 C3H8O2
B) C3H8 + 2 O 3 CH2O + H2O
C) C3H8 + 5 O2 3 CO2 + 4 H2O
D) C3H8 + 4 O CH2O2 + H2O
A) C3H8 + O2 C3H8O2
B) C3H8 + 2 O 3 CH2O + H2O
C) C3H8 + 5 O2 3 CO2 + 4 H2O
D) C3H8 + 4 O CH2O2 + H2O

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
36
The combustion reaction of magnesium results in what compound?
A) MgCl2
B) MgCl4
C) MgO
D) Mg2O3
A) MgCl2
B) MgCl4
C) MgO
D) Mg2O3

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
37
The combustion reaction of calcium results in what compound?
A) CaCl2
B) Ca2O3
C) CaCl4
D) CaO
A) CaCl2
B) Ca2O3
C) CaCl4
D) CaO

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
38
When heated, zinc and sulfur combine to form a new compound, zinc sulfide. In this reaction:
A) both Zn and S are oxidized.
B) both Zn and S are reduced.
C) S is oxidized and Zn is reduced.
D) Zn is oxidized and S is reduced.
A) both Zn and S are oxidized.
B) both Zn and S are reduced.
C) S is oxidized and Zn is reduced.
D) Zn is oxidized and S is reduced.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
39
The equation that shows the applicable dissociation of the reactants and products without the spectator ions is called the _____ equation.
A) net ionic
B) ionic
C) molecular
D) algebraic
A) net ionic
B) ionic
C) molecular
D) algebraic

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
40
According to the solubility rules, which compound is INSOLUBLE in water?
A) Ni(OH)2
B) Li2CO3
C) KBr
D) AgNO3
A) Ni(OH)2
B) Li2CO3
C) KBr
D) AgNO3

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
41
Precipitation reactions and neutralization reactions are both _____ reactions.
A) single displacement
B) double displacement
C) synthesis
D) decomposition
A) single displacement
B) double displacement
C) synthesis
D) decomposition

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
42
Which compound is a base?
A) NaCl
B) KOH
C) HCl
D) H2O
A) NaCl
B) KOH
C) HCl
D) H2O

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
43
Which compound is a salt?
A) NaCl
B) CO
C) CH4
D) H2O
A) NaCl
B) CO
C) CH4
D) H2O

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
44
What is the net ionic equation for the following reaction?
BaCl2 (aq) + Na2CO3 (aq) BaCO3 (s) + 2 NaCl (aq)
A) Ba2+ (aq) + CO32- (aq) BaCO3 (s) + 2 NaCl (s)
B) 2 Na+ (aq) + 2 Cl- (aq) 2 NaCl (aq)
C) Ba2+ (aq) + CO32- (aq) BaCO3 (s)
D) Ba2+ (aq) + 2 Cl- (aq) + 2 Na+ (aq) + CO32- (aq) BaCO3 (s) + 2 Na+ (aq) + 2 Cl- (aq)
BaCl2 (aq) + Na2CO3 (aq) BaCO3 (s) + 2 NaCl (aq)
A) Ba2+ (aq) + CO32- (aq) BaCO3 (s) + 2 NaCl (s)
B) 2 Na+ (aq) + 2 Cl- (aq) 2 NaCl (aq)
C) Ba2+ (aq) + CO32- (aq) BaCO3 (s)
D) Ba2+ (aq) + 2 Cl- (aq) + 2 Na+ (aq) + CO32- (aq) BaCO3 (s) + 2 Na+ (aq) + 2 Cl- (aq)

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
45
Which compound is an acid?
A) NaCl
B) KOH
C) HCl
D) H2O
A) NaCl
B) KOH
C) HCl
D) H2O

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
46
The net ionic equation for any aqueous acid-base neutralization reaction is:
A) H+ (aq) + OH- (aq) H2O (l).
B) Na+ (aq) + Cl- (aq) NaCl (s).
C) 4 H+ (aq) + O2 (g) 2 H2O (l).
D) Na+ (aq) + OH- (aq) NaOH (aq).
A) H+ (aq) + OH- (aq) H2O (l).
B) Na+ (aq) + Cl- (aq) NaCl (s).
C) 4 H+ (aq) + O2 (g) 2 H2O (l).
D) Na+ (aq) + OH- (aq) NaOH (aq).

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
47
What is the net ionic equation for the following reaction?
ZnBr2 (aq) + K2CO3 (aq) 2 KBr (aq) + ZnCO3 (s)
A) Zn2+ (aq) + CO32 (aq) ZnCO3 (s) + 2 KBr (aq)
B) 2 K+ (aq) + 2 Br- (aq) 2 KBr (aq)
C) Zn2+ (aq) + CO32- (aq) ZnCO3 (s)
D) Zn2+ (aq) + 2 Br- (aq) + 2 K+ (aq) + CO32- (aq) 2 Na+ (aq) + 2 Cl- (aq) + ZnCO3 (s)
ZnBr2 (aq) + K2CO3 (aq) 2 KBr (aq) + ZnCO3 (s)
A) Zn2+ (aq) + CO32 (aq) ZnCO3 (s) + 2 KBr (aq)
B) 2 K+ (aq) + 2 Br- (aq) 2 KBr (aq)
C) Zn2+ (aq) + CO32- (aq) ZnCO3 (s)
D) Zn2+ (aq) + 2 Br- (aq) + 2 K+ (aq) + CO32- (aq) 2 Na+ (aq) + 2 Cl- (aq) + ZnCO3 (s)

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck



