Deck 2: The Chemistry of Microbiology
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/76
Play
Full screen (f)
Deck 2: The Chemistry of Microbiology
1
All of the following bases are found in RNA molecules EXCEPT
A) uracil.
B) adenine.
C) guanine.
D) thymine.
E) cytosine.
A) uracil.
B) adenine.
C) guanine.
D) thymine.
E) cytosine.
D
2
DNA is composed of repeating units of sugars, phosphates, and nucleic acids. This is an example of a
A) lipid.
B) polymer.
C) salt.
D) micelle.
E) monomer.
A) lipid.
B) polymer.
C) salt.
D) micelle.
E) monomer.
B
3
Which of the following is an INCORRECT pairing?
A) secondary structure; fi- pleated sheets
B) primary structure; amino acid sequence
C) quaternary structure; two or more polypeptides
D) tertiary structure; covalent bonds
E) secondary structure; disulfide bridges
A) secondary structure; fi- pleated sheets
B) primary structure; amino acid sequence
C) quaternary structure; two or more polypeptides
D) tertiary structure; covalent bonds
E) secondary structure; disulfide bridges
E
4
A protein is a of amino acids.
A) bilayer
B) decomposition product
C) monomer
D) solution
E) polymer
A) bilayer
B) decomposition product
C) monomer
D) solution
E) polymer

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
5
The "backbone" of the DNA molecule is composed of
A) pentoses.
B) amino acids.
C) phosphates.
D) alternating phosphates and pentoses.
E) nitrogenous bases.
A) pentoses.
B) amino acids.
C) phosphates.
D) alternating phosphates and pentoses.
E) nitrogenous bases.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following statements concerning nucleic acids is FALSE?
A) Nucleic acid strands are held together by hydrogen bonds between complementary bases.
B) Some viruses have DNA as their genomes.
C) The nucleic acid polymer is composed of peptide bonds.
D) Not all DNA is double stranded.
E) Cytosine is found in all nucleic acid molecules.
A) Nucleic acid strands are held together by hydrogen bonds between complementary bases.
B) Some viruses have DNA as their genomes.
C) The nucleic acid polymer is composed of peptide bonds.
D) Not all DNA is double stranded.
E) Cytosine is found in all nucleic acid molecules.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is found in nucleic acids?
A) carboxylic acid
B) amines
C) R group
D) glycerol
E) purines
A) carboxylic acid
B) amines
C) R group
D) glycerol
E) purines

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following would NOT normally be found as a component of a cell's nucleic acids?
A) thymine deoxyribonucleotides
B) uracil deoxyribonucleotides
C) adenine ribonucleotides
D) cytosine ribonucleotides
E) adenine deoxyribonucleotides
A) thymine deoxyribonucleotides
B) uracil deoxyribonucleotides
C) adenine ribonucleotides
D) cytosine ribonucleotides
E) adenine deoxyribonucleotides

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
9
An acid dissociates in water to release
A) anion(s).
B) hydroxyl group(s).
C) cation(s).
D) hydrogen ion(s).
E) both anions and hydrogen ions.
A) anion(s).
B) hydroxyl group(s).
C) cation(s).
D) hydrogen ion(s).
E) both anions and hydrogen ions.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
10
Tertiary and quaternary structure of proteins involves bonds.
A) ionic
B) hydrogen
C) nonpolar covalent
D) polar covalent
E) ionic, hydrogen, polar, and nonpolar covalent
A) ionic
B) hydrogen
C) nonpolar covalent
D) polar covalent
E) ionic, hydrogen, polar, and nonpolar covalent

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
11
An amine group is removed from an amino acid and bonded to a second compound to form a different amino acid. No other molecules are used or produced. What type of reaction is likely to be involved?
A) a synthesis reaction
B) a hydrolysis reaction
C) an exchange reaction
D) a decomposition reaction
E) The answer cannot be determined for the available information.
A) a synthesis reaction
B) a hydrolysis reaction
C) an exchange reaction
D) a decomposition reaction
E) The answer cannot be determined for the available information.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
12
Nucleic acids, proteins, and complex carbohydrates are all produced by
A) catabolic reactions.
B) hydrolytic reactions.
C) hydrogen bonding.
D) dehydration synthesis.
E) exchange reactions.
A) catabolic reactions.
B) hydrolytic reactions.
C) hydrogen bonding.
D) dehydration synthesis.
E) exchange reactions.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
13
The valence of an atom represents its
A) electronegativity.
B) radioactivity.
C) ability to interact with water.
D) ability to interact with other atoms.
E) ability to attract electrons.
A) electronegativity.
B) radioactivity.
C) ability to interact with water.
D) ability to interact with other atoms.
E) ability to attract electrons.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
14
Lipids found in the membranes of all eukaryotic cells are
A) steroids.
B) phospholipids.
C) triglycerides.
D) polyunsaturated fats.
E) waxes.
A) steroids.
B) phospholipids.
C) triglycerides.
D) polyunsaturated fats.
E) waxes.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following is a particle found in the nucleus of an atom and that has no electrical charge?
A) isotope
B) element
C) neutron
D) electron
E) proton
A) isotope
B) element
C) neutron
D) electron
E) proton

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is an INCORRECT pairing?
A) electrolytes; anions
B) synthesis; endothermic
C) dehydration; anabolism
D) catabolism; exothermic
E) hydrolysis; hydrogen bonds
A) electrolytes; anions
B) synthesis; endothermic
C) dehydration; anabolism
D) catabolism; exothermic
E) hydrolysis; hydrogen bonds

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
17
Proteins contain both acidic and basic R groups, and can therefore function as
A) buffers.
B) energy storage macromolecules.
C) genetic material.
D) catalysts.
E) structural macromolecules.
A) buffers.
B) energy storage macromolecules.
C) genetic material.
D) catalysts.
E) structural macromolecules.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
18
Unstable isotopes can be useful
A) in medical diagnosis.
B) as buffers.
C) catalysts.
D) in vitamins.
E) in the formation of hydrogen bonds.
A) in medical diagnosis.
B) as buffers.
C) catalysts.
D) in vitamins.
E) in the formation of hydrogen bonds.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
19
Organisms use carbohydrates in all of the following ways EXCEPT
A) as a short- term energy source.
B) as a component of cell walls.
C) to keep membranes flexible at low temperatures.
D) as a building block of DNA and RNA molecules.
E) as a long- term energy source.
A) as a short- term energy source.
B) as a component of cell walls.
C) to keep membranes flexible at low temperatures.
D) as a building block of DNA and RNA molecules.
E) as a long- term energy source.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
20
Decomposition reactions are commonly reactions.
A) anabolic
B) exchange
C) dehydration
D) endothermic
E) exothermic
A) anabolic
B) exchange
C) dehydration
D) endothermic
E) exothermic

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following statements about proteins is FALSE?
A) They have multiple levels of structural organization.
B) They can be hydrophobic, hydrophilic, or both.
C) Their primary function is energy storage.
D) They are formed by dehydration synthesis reactions.
E) They are composed of amino acids.
A) They have multiple levels of structural organization.
B) They can be hydrophobic, hydrophilic, or both.
C) Their primary function is energy storage.
D) They are formed by dehydration synthesis reactions.
E) They are composed of amino acids.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
22
A stable atom has in its valence shell.
A) 10 electrons
B) 8 electrons
C) 2 neutrons
D) 4 electrons
E) 8 protons
A) 10 electrons
B) 8 electrons
C) 2 neutrons
D) 4 electrons
E) 8 protons

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
23
A(n) is a compound that dissolves into anions and cations in water.
A) catalyst.
B) buffer
C) base
D) salt
E) acid
A) catalyst.
B) buffer
C) base
D) salt
E) acid

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
24
Matter composed of a single type of atom is known as a(n)
A) element.
B) compound.
C) electron.
D) molecule.
E) mineral.
A) element.
B) compound.
C) electron.
D) molecule.
E) mineral.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following are examples of pyrimidines?
A) uracil and adenine
B) cytosine and thymine
C) cytosine and guanine
D) thymine and adenine
E) thymine and guanine
A) uracil and adenine
B) cytosine and thymine
C) cytosine and guanine
D) thymine and adenine
E) thymine and guanine

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
26
The type(s) of bond produced when atoms with somewhat different electronegativities share electrons is/are
A) a hydrogen bond.
B) a polar covalent bond.
C) a nonpolar covalent bond.
D) an ionic bond.
E) both nonpolar covalent and ionic bonds.
A) a hydrogen bond.
B) a polar covalent bond.
C) a nonpolar covalent bond.
D) an ionic bond.
E) both nonpolar covalent and ionic bonds.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
27
Anna is conducting an experiment using a pH indicator that is red at low pH, green at neutral pH and purple at high pH. She starts with a green solution. When she adds compound X to her solution it turns purple. Then she adds compound Z to the solution and it turns green. She adds more Z, the solution remains green. These observations suggest X is _ _ and Z is .
A) a base; a buffer
B) an acid; a base
C) a base; a strong acid
D) a buffer; a base
E) an acid; a buffer
A) a base; a buffer
B) an acid; a base
C) a base; a strong acid
D) a buffer; a base
E) an acid; a buffer

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
28
Compounds that readily dissociate in water are
A) polar.
B) ionic.
C) nonpolar.
D) either polar or ionic.
E) never polar or ionic.
A) polar.
B) ionic.
C) nonpolar.
D) either polar or ionic.
E) never polar or ionic.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
29
Plant cell walls are composed of held together by .
A) fatty acids; polar covalent bonds
B) peptidoglycan; ionic bonds
C) polysaccharides; hydrogen bonds
D) amino acids; peptide bonds
E) disaccharides; hydrophobic interactions
A) fatty acids; polar covalent bonds
B) peptidoglycan; ionic bonds
C) polysaccharides; hydrogen bonds
D) amino acids; peptide bonds
E) disaccharides; hydrophobic interactions

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
30
All of the following are associated with ATP molecules EXCEPT
A) formation of coenzymes.
B) a recyclable energy supply.
C) high- energy bonds.
D) three phosphate groups.
E) a long- term energy supply.
A) formation of coenzymes.
B) a recyclable energy supply.
C) high- energy bonds.
D) three phosphate groups.
E) a long- term energy supply.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following is NOT a characteristic of saturated fats?
A) They are found in animals.
B) They are a form of stored energy.
C) They are usually solid at room temperature.
D) Their fatty acids pack tightly together.
E) They contain at least one double bond.
A) They are found in animals.
B) They are a form of stored energy.
C) They are usually solid at room temperature.
D) Their fatty acids pack tightly together.
E) They contain at least one double bond.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following is a property of water?
A) It is liquid in a very narrow temperature range.
B) It has a high capacity for heat.
C) It is not a common reactant in metabolic reactions.
D) It is a nonpolar molecule.
E) It is not a good solvent.
A) It is liquid in a very narrow temperature range.
B) It has a high capacity for heat.
C) It is not a common reactant in metabolic reactions.
D) It is a nonpolar molecule.
E) It is not a good solvent.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
33
Hydrogen bonds are found in all of the following EXCEPT
A) between water molecules.
B) in the DNA double helix between nucleotides.
C) between phosphates in ATP.
D) in a- helices.
E) between the R groups of amino acids in proteins.
A) between water molecules.
B) in the DNA double helix between nucleotides.
C) between phosphates in ATP.
D) in a- helices.
E) between the R groups of amino acids in proteins.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
34
Adenosine triphosphate (ATP) is a
A) polymer.
B) lipid.
C) simple carbohydrate.
D) bilayer.
E) monomer.
A) polymer.
B) lipid.
C) simple carbohydrate.
D) bilayer.
E) monomer.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
35
A polymer composed of simple sugars is a(n)
A) triglyceride.
B) protein.
C) glycoprotein.
D) starch.
E) amino acid.
A) triglyceride.
B) protein.
C) glycoprotein.
D) starch.
E) amino acid.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
36
A(n) is an arrangement of atoms found in a variety of macromolecules.
A) functional group
B) salt
C) buffer
D) isotope
E) stereoisomer
A) functional group
B) salt
C) buffer
D) isotope
E) stereoisomer

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
37
Which parts of the atoms interact in a chemical reaction?
A) electrons
B) neutrons
C) ions
D) isotopes
E) protons
A) electrons
B) neutrons
C) ions
D) isotopes
E) protons

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following types of chemical bonds do carbon atoms generally NOT form?
A) polar covalent bonds
B) ionic bonds
C) hydrogen bonds
D) nonpolar covalent bonds
E) neither ionic nor hydrogen bonds
A) polar covalent bonds
B) ionic bonds
C) hydrogen bonds
D) nonpolar covalent bonds
E) neither ionic nor hydrogen bonds

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
39
Amylose is a(n) carbohydrate.
A) nucleotide
B) polymer
C) monomer
D) ionic
E) simple
A) nucleotide
B) polymer
C) monomer
D) ionic
E) simple

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following is an example of a polysaccharide?
A) glycogen
B) sucrose
C) glucose
D) fructose
E) deoxyribose
A) glycogen
B) sucrose
C) glucose
D) fructose
E) deoxyribose

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
41
The sum of all the chemical reactions within an organism is referred to as its (metabolism/physiology).

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
42
The phosphorylation of a protein by ATP is a(n) (exchange/transfer) reaction

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
43
A chemical reaction in which a water molecule is a reactant is known as a(n) (dehydration/hydrolysis) reaction.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
44
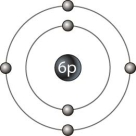 The outer ring in Figure 2- 1 represents
The outer ring in Figure 2- 1 representsA) an isotope.
B) an electron shell.
C) a neutron.
D) an electron.
E) the nucleus.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
45
A hydroxyl acts as a base.
A) anion
B) salt
C) cation
D) group
E) atom
A) anion
B) salt
C) cation
D) group
E) atom

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
46
A(n) (indicator/base/buffer) is a substance that maintains the pH even when the amounts of acid and / or base are changing.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
47
Radioactive iodine is sometimes used to treat thyroid cancer. This is an example of the use of (isotopes/elements/radiation) in medical treatment.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following is NOT a characteristic of phospholipids?
A) They contain fatty acids that associate with water.
B) They contain two fatty acids and a phosphate functional group.
C) They contain a hydrophilic phosphate "head."
D) They are found in cellular membranes.
E) They can form micelles and bilayers.
A) They contain fatty acids that associate with water.
B) They contain two fatty acids and a phosphate functional group.
C) They contain a hydrophilic phosphate "head."
D) They are found in cellular membranes.
E) They can form micelles and bilayers.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
49
The folding of a polypeptide into a three- dimensional shape is its (secondary/tertiary/quaternary) structure.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
50
All of the following are components of an amino acid EXCEPT a(n)
A) a- carbon.
B) R group.
C) carboxyl group.
D) amino group.
E) pentose group.
A) a- carbon.
B) R group.
C) carboxyl group.
D) amino group.
E) pentose group.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
51
A(n) (base/acid) is a molecule that binds with hydrogen ions when it is dissolved in water.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
52
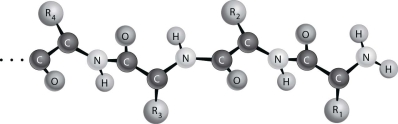
 Figure 2.2 depicts the (primary/secondary/tertiary) structure of a protein.
Figure 2.2 depicts the (primary/secondary/tertiary) structure of a protein.
Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
53
The DNA double helix is held together by (covalent/ionic/hydrogen) bonds.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
54
Cell surface markers composed of both carbohydrate and lipid molecules are known as (glycoproteins/glycolipids/LPS).

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
55
The (atoms/isotopes/stereoisomers) of an element vary in the number of neutrons in the nucleus.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
56
A(n) (catalyst/enzyme) is any molecule that speeds up a chemical reaction.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
57
An atom or molecule becomes a(n) (anion/ion/cation) when it loses an electron to a more electronegative molecule.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
58
The monomer of a nucleic acid is called a (nucleoside/nucleotide/base).

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
59
The reverse of a dehydration synthesis reaction is a(n) reaction.
A) endothermic
B) hydrolytic
C) exchange
D) metabolic
E) anabolic
A) endothermic
B) hydrolytic
C) exchange
D) metabolic
E) anabolic

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
60
The type(s) of bond produced when atoms share electrons equally is/are
A) a nonpolar covalent bond.
B) an ionic bond.
C) a hydrogen bond.
D) a polar covalent bond.
E) both polar covalent and ionic bonds.
A) a nonpolar covalent bond.
B) an ionic bond.
C) a hydrogen bond.
D) a polar covalent bond.
E) both polar covalent and ionic bonds.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
61
The electron shells of atoms hold eight electrons each.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
62
The long- term chemical energy storage molecules in plants are triglycerides.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
63
Max is exploring the properties of various compounds. Some of his explorations involve the use of a pH indicator that is red at low pH, yellow- green at neutral pH and blue to purple at high pH. He sets up several tubes containing water and the pH indicator and then begins to add some of the compounds he is characterizing in various combinations. His results are shown on the Figure 2.3.
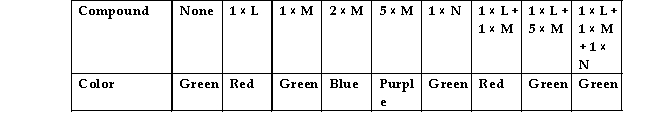 What can Max conclude about his compounds based on these results? Describe the likely events in terms of hydrogen and hydroxyl ions.
What can Max conclude about his compounds based on these results? Describe the likely events in terms of hydrogen and hydroxyl ions.
 What can Max conclude about his compounds based on these results? Describe the likely events in terms of hydrogen and hydroxyl ions.
What can Max conclude about his compounds based on these results? Describe the likely events in terms of hydrogen and hydroxyl ions.
Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
64
Describe the chemical properties of phospholipids that account for their behavior in water.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
65
The side groups of amino acids can interact with each other and with other molecules.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
66
A molecule composed of carbon and hydrogen is a compound.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
67
Hydrogen bonds are stronger then covalent bonds.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
68
Discuss the importance of hydrogen bonds in the chemistry of the cell.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
69
An organic molecule with the chemical formula C4H5O1N3 is probably a pyrimidine.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
70
The smallest chemical units of matter are elements.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
71
Salts are produced from exchange reactions in which acids and bases neutralize each other.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
72
One of the products of dehydration synthesis reactions is water.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
73
Nitrogen is an essential element for living things, as demonstrated by the fact that nearly all fertilizers contain nitrogenous compounds. Discuss why nitrogen is essential.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
74
A chemical reaction that traps energy within newly formed chemical bonds is an (exothermic/endothermic) reaction.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
75
Denaturation of a protein is always permanent.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
76
Compare and contrast synthesis reactions with decomposition reactions.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck



