Deck 2: Atoms, Ions, and Molecules: Matter Starts Here
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/131
Play
Full screen (f)
Deck 2: Atoms, Ions, and Molecules: Matter Starts Here
1
Which statement is not correct?
A) Electrons have a negative electrical charge.
B) Protons have a positive electrical charge.
C) Neutrons do not have an electrical charge.
D) In an atom, the interaction between electrons and protons is attractive.
E) In an atom, the interaction between electrons and neutrons is repulsive.
A) Electrons have a negative electrical charge.
B) Protons have a positive electrical charge.
C) Neutrons do not have an electrical charge.
D) In an atom, the interaction between electrons and protons is attractive.
E) In an atom, the interaction between electrons and neutrons is repulsive.
In an atom, the interaction between electrons and neutrons is repulsive.
2
Which statement best describes isotopes?
A) They have the same atomic mass.
B) They have the same total number of protons and neutrons.
C) They have the same number of neutrons but a different number of protons.
D) They have the same number of protons but a different number of neutrons.
E) They have very different chemical reactivity.
A) They have the same atomic mass.
B) They have the same total number of protons and neutrons.
C) They have the same number of neutrons but a different number of protons.
D) They have the same number of protons but a different number of neutrons.
E) They have very different chemical reactivity.
They have the same total number of protons and neutrons.
3
Which statement about isotopes of the same element is not correct?
A) They have the same number of protons.
B) They have different numbers of neutrons.
C) They have essentially the same chemical properties.
D) They have the same atomic mass.
E) They have the same number of electrons.
A) They have the same number of protons.
B) They have different numbers of neutrons.
C) They have essentially the same chemical properties.
D) They have the same atomic mass.
E) They have the same number of electrons.
They have the same atomic mass.
4
Which particle-level diagram is the best representation of a ion? 
A)
B)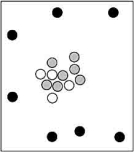
C)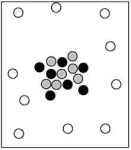
D)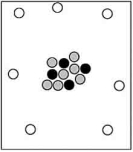

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
5
If an atom had a radius of 100 m, it would be approximately the size of a football stadium. On this scale, what would be the radius of the atomic nucleus? The radius of the nucleus is approximately 10,000 times smaller than the radius of an atom.
A) 1 mm, like a very dull pencil point
B) 1 cm, like a dime
C) 10 cm, like your longest finger
D) 10 m, like a red blood cell
E) 100 pm, like a real atom
A) 1 mm, like a very dull pencil point
B) 1 cm, like a dime
C) 10 cm, like your longest finger
D) 10 m, like a red blood cell
E) 100 pm, like a real atom

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
6
Which statement is not correct? In atomic mass units (amu or u), __________
A) the mass of an electron, proton, or neutron is approximately 1 u.
B) the mass of a proton or neutron is approximately 1 u, and the mass of an electron is approximately 0 u.
C) the mass of an atom is approximately equal to the number of protons and neutrons in the nucleus of the atom.
D) the mass of a carbon-12 atom is exactly 12 u.
E) the mass of an oxygen-16 atom is approximately 16 u.
A) the mass of an electron, proton, or neutron is approximately 1 u.
B) the mass of a proton or neutron is approximately 1 u, and the mass of an electron is approximately 0 u.
C) the mass of an atom is approximately equal to the number of protons and neutrons in the nucleus of the atom.
D) the mass of a carbon-12 atom is exactly 12 u.
E) the mass of an oxygen-16 atom is approximately 16 u.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
7
Which particle-level diagram is the best representation for a 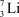 atom?
atom? 
A)
B)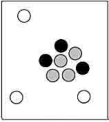
C)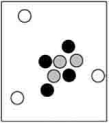
D)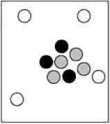
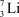 atom?
atom? 
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
8
Which statement is correct?
A) Electrons, protons, and neutrons have about the same mass.
B) Electrons have a much smaller mass than that of protons and neutrons.
C) Neutrons are much more massive than protons.
D) Protons are much more massive than neutrons.
E) Electrons have a much larger mass than that of protons and neutrons.
A) Electrons, protons, and neutrons have about the same mass.
B) Electrons have a much smaller mass than that of protons and neutrons.
C) Neutrons are much more massive than protons.
D) Protons are much more massive than neutrons.
E) Electrons have a much larger mass than that of protons and neutrons.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
9
Who discovered electrons?
A) Robert Boyle
B) Robert Millikan
C) Joseph John Thomson
D) John Dalton
E) Albert Einstein
A) Robert Boyle
B) Robert Millikan
C) Joseph John Thomson
D) John Dalton
E) Albert Einstein

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
10
What is the correct symbol for a proton?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
11
What is the correct symbol for a neutron?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
12
What is the correct symbol for an electron?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
13
Rutherford, Geiger, and Marsden's experiment demonstrated that the volume of the nucleus is roughly what fraction of the volume occupied by the electrons?
A) 1/10
B) 1/100
C) 1/1,000
D) 1/10,000
E) 1/100,000
A) 1/10
B) 1/100
C) 1/1,000
D) 1/10,000
E) 1/100,000

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
14
Protons and neutrons are examples of __________
A) nuclei.
B) nuclides.
C) nucleons.
D) isotopes.
E) charged particles.
A) nuclei.
B) nuclides.
C) nucleons.
D) isotopes.
E) charged particles.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
15
Who was the first scientist to determine the charge of an electron?
A) Robert Boyle
B) Robert Millikan
C) Joseph John Thomson
D) John Dalton
E) Albert Einstein
A) Robert Boyle
B) Robert Millikan
C) Joseph John Thomson
D) John Dalton
E) Albert Einstein

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
16
When cosmic rays strike atoms in the upper atmosphere, energetic neutrons are produced. These neutrons collide with nitrogen-14 atoms, producing carbon-14 atoms and hydrogen atoms. Which diagram represents the carbon-14 product? 
A)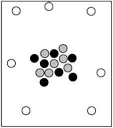
B)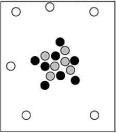
C)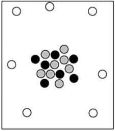
D)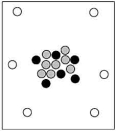

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
17
The 4He nucleus is an example of __________
A) a nuclide.
B) an element.
C) a proton.
D) a neutron.
E) a nucleon.
A) a nuclide.
B) an element.
C) a proton.
D) a neutron.
E) a nucleon.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
18
Which one of the following experiments provided evidence that atoms contained small massive nuclei with positive charges?
A) Bunsen and Kirchoff's flame test
B) Fraunhofer lines
C) the Rutherford-Geiger-Marsden experiment
D) Thomson's experiments with cathode ray tubes
E) Millikan's oil-drop experiment
A) Bunsen and Kirchoff's flame test
B) Fraunhofer lines
C) the Rutherford-Geiger-Marsden experiment
D) Thomson's experiments with cathode ray tubes
E) Millikan's oil-drop experiment

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
19
In the atoms in the Rutherford-Geiger-Marsden experiment, the alpha particles were repelled by __________
A) electrons.
B) protons.
C) neutrons.
D) nuclei.
E) gravity.
A) electrons.
B) protons.
C) neutrons.
D) nuclei.
E) gravity.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
20
In the Rutherford-Geiger-Marsden experiment, alpha particles were projected at a thin film of __________
A) gold.
B) silver.
C) platinum.
D) sodium.
E) aluminum.
A) gold.
B) silver.
C) platinum.
D) sodium.
E) aluminum.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
21
Enriched weapons-grade uranium consists of 80% uranium-235 (235.044 amu) and 20% uranium-238 (238.051 amu). What is the average atomic mass of weapons-grade uranium, assuming the percentages are exact?
A) 235.044 amu
B) 236.547 amu
C) 238.051 amu
D) 235.645 amu
E) 235.754 amu
A) 235.044 amu
B) 236.547 amu
C) 238.051 amu
D) 235.645 amu
E) 235.754 amu

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
22
A atom has __________ protons, __________ neutrons, and __________ electrons.
A) 17, 18, 19
B) 17, 20, 17
C) 17, 17, 20
D) 17, 18, 17
E) 18, 17, 18
A) 17, 18, 19
B) 17, 20, 17
C) 17, 17, 20
D) 17, 18, 17
E) 18, 17, 18

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
23
What is the symbol for magnesium?
A) M
B) Mg
C) Mn
D) Mo
E) Ma
A) M
B) Mg
C) Mn
D) Mo
E) Ma

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
24
An unknown element is found to contain isotopes with the following masses and natural abundances: 38.9637 amu (93.08%), 39.9640 amu (0.012%), and 40.9618 amu (6.91%). Using these data, identify the element.
A) S
B) Cl
C) Ar
D) K
E) Ca
A) S
B) Cl
C) Ar
D) K
E) Ca

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
25
The Curiosity rover now on Mars analyzed rocks and found magnesium to have the following isotopic composition. What is the average atomic mass of magnesium in these rocks?
A) 24.31 u
B) 24.29 u
C) 24.33 u
D) 24.99 u
E) 33.33 u
A) 24.31 u
B) 24.29 u
C) 24.33 u
D) 24.99 u
E) 33.33 u

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
26
Technetium often is used to image areas of bone growth because it is a radioisotope with a half-life of 6 hours that emits gamma rays. A ion has __________ protons, __________ neutrons, and __________electrons.
A) 99, 43, 98
B) 43, 99, 42
C) 43, 56, 42
D) 56, 43, 43
E) 43, 99, 43
A) 99, 43, 98
B) 43, 99, 42
C) 43, 56, 42
D) 56, 43, 43
E) 43, 99, 43

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
27
Tellurium and iodine appear next to each other in the periodic table with tellurium in group 16 and iodine in group 17. Mendeleev's early periodic table had this order reversed because __________
A) the mass number is larger for iodine.
B) tellurium has one more proton than iodine.
C) iodine has one more proton than tellurium.
D) Te comes after I in the alphabet.
E) in total tellurium has more protons and neutrons than iodine.
A) the mass number is larger for iodine.
B) tellurium has one more proton than iodine.
C) iodine has one more proton than tellurium.
D) Te comes after I in the alphabet.
E) in total tellurium has more protons and neutrons than iodine.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
28
Which statement regarding the organization of the periodic table is not correct?
A) Mendeleev arranged known elements with similar chemical properties in columns.
B) Mendeleev's predictions of the chemical properties of unknown elements facilitated their discovery.
C) Mendeleev arranged the elements in order of increasing atomic mass.
D) The modern periodic table arranges elements in order of increasing atomic number.
E) The elements go from gases to liquids to solids in order down the columns in Mendeleev's periodic table.
A) Mendeleev arranged known elements with similar chemical properties in columns.
B) Mendeleev's predictions of the chemical properties of unknown elements facilitated their discovery.
C) Mendeleev arranged the elements in order of increasing atomic mass.
D) The modern periodic table arranges elements in order of increasing atomic number.
E) The elements go from gases to liquids to solids in order down the columns in Mendeleev's periodic table.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
29
What is the symbol for sulfur?
A) Si
B) Sc
C) Su
D) S
E) Sf
A) Si
B) Sc
C) Su
D) S
E) Sf

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
30
A ion has __________ protons, __________ neutrons, and __________ electrons.
A) 8, 8, 6
B) 8, 10, 10
C) 8, 8, 10
D) 8, 8, 8
E) 8, 16, 8
A) 8, 8, 6
B) 8, 10, 10
C) 8, 8, 10
D) 8, 8, 8
E) 8, 16, 8

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
31
A hypothetical element has two stable isotopes: one isotope has a mass of 106.9051 amu with an abundance of 48.183%, the other isotope has a mass of 108.9048 amu with an abundance of 51.825%. What is the average atomic mass of this element?
A) 107.980 amu
B) 107.970 amu
C) 107.960 amu
D) 107.950 amu
E) 107.940 amu
A) 107.980 amu
B) 107.970 amu
C) 107.960 amu
D) 107.950 amu
E) 107.940 amu

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
32
Zinc has five naturally occurring isotopes with an average mass of 65.39 amu. Three isotopes, in roughly equal amounts, account for 95% of zinc. Which isotope is most abundant?
A) (64Zn, 63.9291 amu)
B) (66Zn, 65.9260 amu)
C) (67Zn, 66.9271 amu)
D)(68Zn, 67.9249 amu)
E) (70Zn, 69.9253 amu)
A) (64Zn, 63.9291 amu)
B) (66Zn, 65.9260 amu)
C) (67Zn, 66.9271 amu)
D)(68Zn, 67.9249 amu)
E) (70Zn, 69.9253 amu)

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
33
The average atomic mass of lithium is 6.941 amu. Lithium has two naturally occurring isotopes, 6Li (7.52%) and 7Li (92.48%). The mass of 6Li is 6.0151 amu. What is the mass of 7Li?
A) 7.016 amu
B) 0.926 amu
C) 6.001 amu
D) 7.000 amu
E) 6.941 amu
A) 7.016 amu
B) 0.926 amu
C) 6.001 amu
D) 7.000 amu
E) 6.941 amu

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
34
What is the symbol of the ion having 12 protons and 10 electrons?
A) Mg2+
B) Al3+
C) Mg2-
D) Na2+
E) Mg
A) Mg2+
B) Al3+
C) Mg2-
D) Na2+
E) Mg

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
35
For each of the elements below, there are only two naturally occurring isotopes. Using information in your periodic table, identify the pair in which the heavier isotope is the more abundant one.
A) (63Cu and 65Cu)
B) (85Rb and 87Rb)
C) (10B and 11B)
D) (79Br and 81Br)
E) (14N and 15N)
A) (63Cu and 65Cu)
B) (85Rb and 87Rb)
C) (10B and 11B)
D) (79Br and 81Br)
E) (14N and 15N)

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
36
Tellurium and iodine appear next to each other in the periodic table. Tellurium is in group 16 and iodine is in group 17 because __________.
A) the mass number is larger for iodine.
B) tellurium has one more proton than iodine.
C) iodine has one more proton than tellurium.
D) the mass number is larger for tellurium.
E) Mendeleev identified that this order is the correct one.
A) the mass number is larger for iodine.
B) tellurium has one more proton than iodine.
C) iodine has one more proton than tellurium.
D) the mass number is larger for tellurium.
E) Mendeleev identified that this order is the correct one.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
37
The mass of thallium (Tl) on the periodic table is given as 204.3833 without any units. There are 47 isotopes of thallium, but only two are stable and abundant, thallium-203, with a mass of 202.9723 amu, and thallium-205, with a mass of 204.9744 amu. What is the percentage of each of these isotopes in naturally occurring thallium?
A) 29.5% 203Tl and 70.5% 205Tl
B) 70.5% 203Tl and 29.5% 205Tl
C) 25.5% 203Tl and 74.5% 205Tl
D) 74.5% 203Tl and 25.5% 205Tl
E) 32.5% 203Tl and 67.5% 205Tl
A) 29.5% 203Tl and 70.5% 205Tl
B) 70.5% 203Tl and 29.5% 205Tl
C) 25.5% 203Tl and 74.5% 205Tl
D) 74.5% 203Tl and 25.5% 205Tl
E) 32.5% 203Tl and 67.5% 205Tl

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
38
The average atomic mass of zinc is 65.39 amu. Given the data in the following table, what is the natural abundance of 66Zn?
A) 27.83%
B) 0.2783%
C) 50.00%
D) 2.783%
E) 28.73%
A) 27.83%
B) 0.2783%
C) 50.00%
D) 2.783%
E) 28.73%

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
39
The average atomic mass of calcium is 40.078 amu. Which is likely to be the most abundant isotope of calcium?
A) (40Ca)
B) (42Ca)
C) (43Ca)
D) (44Ca)
E) (48Ca)
A) (40Ca)
B) (42Ca)
C) (43Ca)
D) (44Ca)
E) (48Ca)

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
40
For each of the elements below, there are only two naturally occurring isotopes. Using information in your periodic table, identify the pair in which the lighter isotope is the more abundant one.
A) (6Li and 7Li)
B) (79Br and 81Br)
C) (10B and 11B)
D) (191Ir and 193Ir)
E) (50V and 51V)
A) (6Li and 7Li)
B) (79Br and 81Br)
C) (10B and 11B)
D) (191Ir and 193Ir)
E) (50V and 51V)

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
41
He is the symbol for __________
A) hydrogen.
B) hafnium.
C) mercury.
D) helium.
E) holmium.
A) hydrogen.
B) hafnium.
C) mercury.
D) helium.
E) holmium.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
42
Silicon is best described as a __________
A) metalloid.
B) metal.
C) transition metal.
D) noble gas.
E) nonmetal.
A) metalloid.
B) metal.
C) transition metal.
D) noble gas.
E) nonmetal.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following is an alkali metal?
A) K
B) Mg
C) Al
D) Cu
E) Ca
A) K
B) Mg
C) Al
D) Cu
E) Ca

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
44
Identify the statement regarding O2, NO, and H2O that is correct.
A) Only O2 is a chemical element.
B) Only NO is a chemical element.
C) Only H2O is a chemical compound.
D) All are chemical elements.
E) All are chemical compounds.
A) Only O2 is a chemical element.
B) Only NO is a chemical element.
C) Only H2O is a chemical compound.
D) All are chemical elements.
E) All are chemical compounds.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
45
Potassium is best described as a __________
A) metalloid.
B) metal.
C) transition metal.
D) noble gas.
E) nonmetal.
A) metalloid.
B) metal.
C) transition metal.
D) noble gas.
E) nonmetal.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
46
Oxygen is best described as a __________
A) metalloid.
B) metal.
C) transition metal.
D) noble gas.
E) nonmetal.
A) metalloid.
B) metal.
C) transition metal.
D) noble gas.
E) nonmetal.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
47
Which statement below is not correct?
A) Oxygen is a chalcogen.
B) Calcium is an alkaline earth metal.
C) Silicon is a nonmetal.
D) Sodium is an alkali metal.
E) Bromine is a halogen.
A) Oxygen is a chalcogen.
B) Calcium is an alkaline earth metal.
C) Silicon is a nonmetal.
D) Sodium is an alkali metal.
E) Bromine is a halogen.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
48
Iron can form two sulfides: FeS and Fe2S3. Use Dalton's law of multiple proportions to predict the ratio of the two masses of sulfur that combine with 100 g of iron in each case to form these compounds.
A) 1:1
B) 1:3
C) 1:2
D) 2:3
E) 3:4
A) 1:1
B) 1:3
C) 1:2
D) 2:3
E) 3:4

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
49
Cobalt is best described as a(n) __________
A) metalloid.
B) transition metal.
C) chalcogen.
D) alkaline earth metal.
E) nonmetal.
A) metalloid.
B) transition metal.
C) chalcogen.
D) alkaline earth metal.
E) nonmetal.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
50
Elements in group 18 (VIIIA) are called __________
A) alkali metals.
B) noble gases.
C) alkaline earth metals.
D) halogens.
E) chalcogens.
A) alkali metals.
B) noble gases.
C) alkaline earth metals.
D) halogens.
E) chalcogens.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
51
Nitrogen and oxygen combine to form several different nitrogen oxides. In one case, 8.4 g of nitrogen reacted completely with 4.8 g of oxygen. In another case, 4.2 g of nitrogen reacted with 9.6 g of oxygen. Which pair of nitrogen oxides is consistent with these data?
A) NO and N2O
B) NO and NO2
C) N2O and N2O5
D) NO and N2O4
E) N2O and N2O4
A) NO and N2O
B) NO and NO2
C) N2O and N2O5
D) NO and N2O4
E) N2O and N2O4

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
52
When 10.0 g of sulfur is combined with 10.0 g of oxygen, 20.0 g of sulfur dioxide is formed. What mass of oxygen would be required to convert 10.0 g of sulfur into sulfur trioxide?
A) 5.0 g
B) 10 g
C) 15 g
D) 30 g
E) 20 g
A) 5.0 g
B) 10 g
C) 15 g
D) 30 g
E) 20 g

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
53
Dalton's law of multiple proportions can be applied to __________
A) H2O and CO2.
B) CO and NO.
C) PF3 and PF5.
D) SO2 and CO.
E) O2 and O3.
A) H2O and CO2.
B) CO and NO.
C) PF3 and PF5.
D) SO2 and CO.
E) O2 and O3.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
54
Which one of the following statements is not consistent with Dalton's atomic view of matter?
A) Atoms of one element can be converted into atoms of another element.
B) Each element is composed of atoms that are identical in size, mass, and chemical properties.
C) Compounds are formed from different atoms in simple whole number ratios.
D) Atoms of different elements can combine in several different proportions to make different compounds.
E) Matter is discrete, as proposed by Democritus.
A) Atoms of one element can be converted into atoms of another element.
B) Each element is composed of atoms that are identical in size, mass, and chemical properties.
C) Compounds are formed from different atoms in simple whole number ratios.
D) Atoms of different elements can combine in several different proportions to make different compounds.
E) Matter is discrete, as proposed by Democritus.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
55
The elements below are used in fireworks. Which one is not classified correctly according to its position in the periodic table?
A) Sodium is an alkali metal.
B) Strontium is an alkaline earth metal.
C) Iron is a transition metal.
D) Phosphorus is a nonmetal.
E) Sulfur is a metalloid.
A) Sodium is an alkali metal.
B) Strontium is an alkaline earth metal.
C) Iron is a transition metal.
D) Phosphorus is a nonmetal.
E) Sulfur is a metalloid.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
56
Calcium is an example of __________
A) an alkali metal.
B) a transition metal.
C) an alkaline earth metal.
D) a halogen.
E) a chalcogen.
A) an alkali metal.
B) a transition metal.
C) an alkaline earth metal.
D) a halogen.
E) a chalcogen.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
57
Dalton's law of multiple proportions deals with __________
A) the proportions of reacting chemicals that maximize the reaction rate.
B) the total number of different compounds that can be made from two elements.
C) the volumes of two elements that can combine to form two or more compounds.
D) the relative masses of two elements that can combine to form two or more compounds.
E) reactions that involve multiple steps.
A) the proportions of reacting chemicals that maximize the reaction rate.
B) the total number of different compounds that can be made from two elements.
C) the volumes of two elements that can combine to form two or more compounds.
D) the relative masses of two elements that can combine to form two or more compounds.
E) reactions that involve multiple steps.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
58
Elements in group 17 (VIIA) are called __________
A) alkali metals.
B) pnictogens.
C) alkaline earth metal.
D) halogens.
E) chalcogens.
A) alkali metals.
B) pnictogens.
C) alkaline earth metal.
D) halogens.
E) chalcogens.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
59
The sixth period of the periodic table contains __________ elements.
A) 18
B) 32
C) 24
D) 16
E) 8
A) 18
B) 32
C) 24
D) 16
E) 8

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
60
Elements 21-30 are known as __________
A) alkaline earths.
B) chalcogens.
C) halides.
D) transition metals.
E) rare earths.
A) alkaline earths.
B) chalcogens.
C) halides.
D) transition metals.
E) rare earths.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
61
Which one of the following is a cation?
A) NO3-
B) SO2
C) Ca2+
D) Na
E) O2
A) NO3-
B) SO2
C) Ca2+
D) Na
E) O2

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
62
Identify the binary compound that has ionic bonding.
A) H2O
B) NO
C) LiF
D) CH4
E) CF4
A) H2O
B) NO
C) LiF
D) CH4
E) CF4

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
63
What is the correct formula for the compound formed between sodium and iodine based on their positions in the periodic table?
A) Na2I
B) NaI2
C) NaI
D) Na2I2
E) Na3I
A) Na2I
B) NaI2
C) NaI
D) Na2I2
E) Na3I

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
64
Which element labeled A-E in the periodic table below will have an ionic charge of -1? 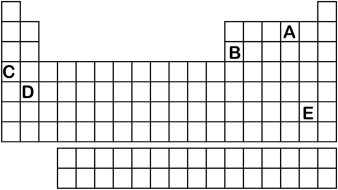
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
65
What is the empirical formula for benzene, C6H6?
A) CH
B) C6H6
C) C2H2
D) CH3
E) C6H
A) CH
B) C6H6
C) C2H2
D) CH3
E) C6H

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
66
How many atoms of each element are there in the compound (NH4)2(S2O3)·5H2O?
A) nitrogen 2, hydrogen 18, sulfur 2, oxygen 8
B) nitrogen 1, hydrogen 6, sulfur 2, oxygen 4
C) nitrogen 2, hydrogen 10, sulfur 2, oxygen 4
D) nitrogen 1, hydrogen 6, sulfur 2, oxygen 8
E) nitrogen 2, hydrogen 8, sulfur 2, oxygen 3
A) nitrogen 2, hydrogen 18, sulfur 2, oxygen 8
B) nitrogen 1, hydrogen 6, sulfur 2, oxygen 4
C) nitrogen 2, hydrogen 10, sulfur 2, oxygen 4
D) nitrogen 1, hydrogen 6, sulfur 2, oxygen 8
E) nitrogen 2, hydrogen 8, sulfur 2, oxygen 3

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
67
Which element labeled A-E in the periodic table below will have an ionic charge of +1? 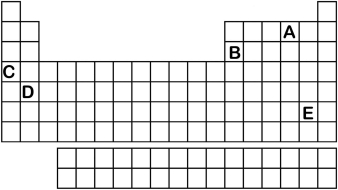
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following is most likely to exhibit covalent bonding?
A) NaF
B) CaCl2
C) Cs2O
D) CO2
E) NaCl
A) NaF
B) CaCl2
C) Cs2O
D) CO2
E) NaCl

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
69
Which element labeled A-E in the periodic table below will have an ionic charge of -2? 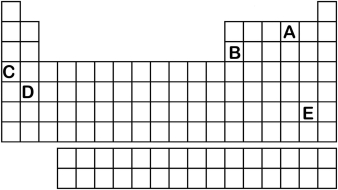
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
70
How many atoms of each element are there in the compound Na3(PO4)3?
A) sodium 3, phosphorus 3, oxygen 12
B) sodium 9, phosphorus 3, oxygen 12
C) sodium 3, phosphorus 1, oxygen 4
D) sodium 3, potassium 1, oxygen 4
E) sodium 9, potassium 3, oxygen 12
A) sodium 3, phosphorus 3, oxygen 12
B) sodium 9, phosphorus 3, oxygen 12
C) sodium 3, phosphorus 1, oxygen 4
D) sodium 3, potassium 1, oxygen 4
E) sodium 9, potassium 3, oxygen 12

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
71
Locate each element in the periodic table and identify which statement is not correct. The common ion of __________ has __________ electrons and a charge of __________.
A) Cs, 55, +1
B) Ca, 18, +2
C) Ba, 54, +2
D) S, 18, -2
E) Cl, 18, -1
A) Cs, 55, +1
B) Ca, 18, +2
C) Ba, 54, +2
D) S, 18, -2
E) Cl, 18, -1

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
72
Locate each element in the periodic table and identify which statement is not correct. The common ion of __________ has __________ electrons and a charge of __________.
A) Na, 10, +1
B) K, 18, +1
C) Mg, 10, +2
D) O, 10, -2
E) F, 10, -2
A) Na, 10, +1
B) K, 18, +1
C) Mg, 10, +2
D) O, 10, -2
E) F, 10, -2

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
73
Which element labeled A-E in the periodic table below will have an ionic charge of +3? 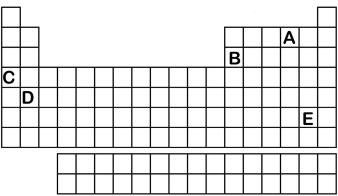
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
74
Based on the element's position in the periodic table, which statement below is not correct?
A) The charge on an ion of sodium is 1+.
B) The charge on an ion of magnesium is 2+.
C) The charge on an ion of oxygen is 2-.
D) The charge on an ion of chlorine is 1-.
E) Ca2+ has more electrons than Ar.
A) The charge on an ion of sodium is 1+.
B) The charge on an ion of magnesium is 2+.
C) The charge on an ion of oxygen is 2-.
D) The charge on an ion of chlorine is 1-.
E) Ca2+ has more electrons than Ar.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
75
Based on its position in the periodic table, which atom would you predict to form an ionic compound with two bromine atoms?
A) sodium
B) aluminum
C) lithium
D) calcium
E) carbon
A) sodium
B) aluminum
C) lithium
D) calcium
E) carbon

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
76
Based on its position in the periodic table, which atom would you predict to form a compound with one chlorine atom?
A) boron
B) aluminum
C) lithium
D) calcium
E) carbon
A) boron
B) aluminum
C) lithium
D) calcium
E) carbon

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
77
Which one of the following is a molecular compound? Molecular compounds also are known as covalent compounds.
A) Na2O
B) CaO
C) FeO
D) CCl4
E) Fe2O3
A) Na2O
B) CaO
C) FeO
D) CCl4
E) Fe2O3

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
78
Which one of the following is an ionic compound?
A) SO2
B) ClO2
C) H2O
D) TiO2
E) CO2
A) SO2
B) ClO2
C) H2O
D) TiO2
E) CO2

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
79
Which element labeled A-E in the periodic table below will have an ionic charge of +2? 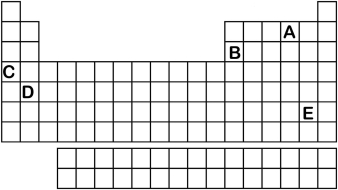
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
80
Which one of the following is an anion?
A) Na+
B) CO2
C) Cl-
D) Na
E) O3
A) Na+
B) CO2
C) Cl-
D) Na
E) O3

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck



