Deck 18: Acid-Base Equilibria
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/106
Play
Full screen (f)
Deck 18: Acid-Base Equilibria
1
Which, if any, of the following acids is strong?
A) phosphoric
B) carbonic
C) acetic
D) water
E) None of these choices is correct.
A) phosphoric
B) carbonic
C) acetic
D) water
E) None of these choices is correct.
None of these choices is correct.
2
The substance NaNO3 is considered
A) a weak Arrhenius acid.
B) a weak Arrhenius base.
C) a strong Arrhenius acid.
D) a strong Arrhenius base.
E) a neutral compound.
A) a weak Arrhenius acid.
B) a weak Arrhenius base.
C) a strong Arrhenius acid.
D) a strong Arrhenius base.
E) a neutral compound.
a neutral compound.
3
Which one of the following will give a solution with a pH > 7, but is not an Arrhenius base in the strict sense?
A) CH3NH2
B) NaOH
C) CO2
D) Ca(OH)2
E) CH4
A) CH3NH2
B) NaOH
C) CO2
D) Ca(OH)2
E) CH4
CH3NH2
4
Given: 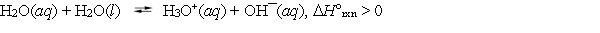 When the temperature of a sample of pure water is raised above 25 C,
When the temperature of a sample of pure water is raised above 25 C,
A) the hydronium ion concentration will be greater than the hydroxide ion concentration.
B) the hydronium ion concentration will be less than the hydroxide ion concentration.
C) the value of Kw will increase.
D) the hydronium ion concentration could change to 1.0 *10¯10 M.
E) the hydroxide ion concentration could change to 1.0 *10¯10 M.
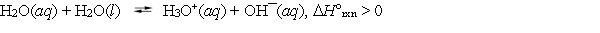 When the temperature of a sample of pure water is raised above 25 C,
When the temperature of a sample of pure water is raised above 25 C,A) the hydronium ion concentration will be greater than the hydroxide ion concentration.
B) the hydronium ion concentration will be less than the hydroxide ion concentration.
C) the value of Kw will increase.
D) the hydronium ion concentration could change to 1.0 *10¯10 M.
E) the hydroxide ion concentration could change to 1.0 *10¯10 M.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following is the strongest base?
A) CH3NH2
B) NaNO3
C) B(OH)3
D) Al(OH)3
E) LiOH
A) CH3NH2
B) NaNO3
C) B(OH)3
D) Al(OH)3
E) LiOH

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following pairs has the stronger acid listed first?
A) HBr, HI
B) HClO2, HClO3
C) H2SeO4, H2SeO3
D) HNO2, HNO3
E) HF, HCl
A) HBr, HI
B) HClO2, HClO3
C) H2SeO4, H2SeO3
D) HNO2, HNO3
E) HF, HCl

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
7
The substance Ca(OH)2 is considered
A) a weak Arrhenius acid.
B) a weak Arrhenius base.
C) a strong Arrhenius acid.
D) a strong Arrhenius base.
E) a neutral compound.
A) a weak Arrhenius acid.
B) a weak Arrhenius base.
C) a strong Arrhenius acid.
D) a strong Arrhenius base.
E) a neutral compound.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
8
Select the strongest acid from the following list.
A) HBrO4
B) HClO
C) HBrO2
D) HBrO
E) HIO
A) HBrO4
B) HClO
C) HBrO2
D) HBrO
E) HIO

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
9
The substance Ba(OH)2 is considered
A) a weak Arrhenius acid.
B) a weak Arrhenius base.
C) a strong Arrhenius acid.
D) a strong Arrhenius base.
E) a neutral compound.
A) a weak Arrhenius acid.
B) a weak Arrhenius base.
C) a strong Arrhenius acid.
D) a strong Arrhenius base.
E) a neutral compound.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is the strongest acid?
A) CH3COOH
B) HF
C) H3PO4
D) H2SO3
E) HI
A) CH3COOH
B) HF
C) H3PO4
D) H2SO3
E) HI

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
11
The substance NH3 is considered
A) a weak acid.
B) a weak base.
C) a strong acid.
D) a strong base.
E) a neutral compound.
A) a weak acid.
B) a weak base.
C) a strong acid.
D) a strong base.
E) a neutral compound.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is the strongest acid?
A) CH3COOH
B) HF
C) H3PO4
D) H2SO3
E) H2SO4
A) CH3COOH
B) HF
C) H3PO4
D) H2SO3
E) H2SO4

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
13
Select the strongest acid from the following list.
A) HBrO
B) HBrO2
C) HClO2
D) HClO3
E) HIO
A) HBrO
B) HBrO2
C) HClO2
D) HClO3
E) HIO

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
14
The substance H2SO3 is considered
A) a weak Arrhenius base.
B) a strong Arrhenius acid.
C) a strong Arrhenius base.
D) a neutral compound.
E) a weak Arrhenius acid.
A) a weak Arrhenius base.
B) a strong Arrhenius acid.
C) a strong Arrhenius base.
D) a neutral compound.
E) a weak Arrhenius acid.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
15
The substance HOBr is considered
A) a weak Arrhenius acid.
B) a weak Arrhenius base.
C) a strong Arrhenius acid.
D) a strong Arrhenius base.
E) a neutral compound.
A) a weak Arrhenius acid.
B) a weak Arrhenius base.
C) a strong Arrhenius acid.
D) a strong Arrhenius base.
E) a neutral compound.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
16
The substance (CH3CH2)2NH is considered
A) a weak acid.
B) a weak base.
C) a strong acid.
D) a strong base.
E) a neutral compound.
A) a weak acid.
B) a weak base.
C) a strong acid.
D) a strong base.
E) a neutral compound.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
17
The substance HClO4 is considered
A) a weak acid.
B) a weak base.
C) a strong acid.
D) a strong base.
E) a neutral compound.
A) a weak acid.
B) a weak base.
C) a strong acid.
D) a strong base.
E) a neutral compound.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
18
The substance HCl is considered
A) a weak Arrhenius acid.
B) a weak Arrhenius base.
C) a strong Arrhenius acid.
D) a strong Arrhenius base.
E) a neutral compound.
A) a weak Arrhenius acid.
B) a weak Arrhenius base.
C) a strong Arrhenius acid.
D) a strong Arrhenius base.
E) a neutral compound.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
19
Which one of the following is a strong acid?
A) H2CO3
B) H2SO3
C) H2SO4
D) H3PO4
E) CH3COOH
A) H2CO3
B) H2SO3
C) H2SO4
D) H3PO4
E) CH3COOH

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following pairs has the stronger acid listed first?
A) H2AsO3, H2AsO4
B) HI, HBr
C) HClO, HClO3
D) H2S, HCl
E) H2SO3, H2SO4
A) H2AsO3, H2AsO4
B) HI, HBr
C) HClO, HClO3
D) H2S, HCl
E) H2SO3, H2SO4

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
21
What is the [OH¯] for a solution at 25 C that has [H3O+] = 8.23 *10¯2 M?
A) > 10¯5 M
B) 1.22 * 10¯6 M
C) 8.23 *10¯12 M
D) 1.22 * 10¯13 M
E) 8.23 * 10¯16 M
A) > 10¯5 M
B) 1.22 * 10¯6 M
C) 8.23 *10¯12 M
D) 1.22 * 10¯13 M
E) 8.23 * 10¯16 M

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following aqueous systems has the highest pH?
A) 0.1 M HA, pKa = 11.89
B) 0.1 M HMO, pKa = 8.23
C) 0.1 M HA, pKa = 4.55
D) 0.1 M HBO, pKa = 2.43
E) pure water
A) 0.1 M HA, pKa = 11.89
B) 0.1 M HMO, pKa = 8.23
C) 0.1 M HA, pKa = 4.55
D) 0.1 M HBO, pKa = 2.43
E) pure water

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
23
What is the pH of a 0.0035 M KOH solution?
A) 2.46
B) 5.65
C) 8.35
D) 11.54
E) None of these choices is correct.
A) 2.46
B) 5.65
C) 8.35
D) 11.54
E) None of these choices is correct.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following acids has the lowest pH?
A) 0.1 M HBO, pKa = 2.43
B) 0.1 M HA, pKa = 4.55
C) 0.1 M HMO, pKa = 8.23
D) 0.1 M HST, pKa = 11.89
E) pure water
A) 0.1 M HBO, pKa = 2.43
B) 0.1 M HA, pKa = 4.55
C) 0.1 M HMO, pKa = 8.23
D) 0.1 M HST, pKa = 11.89
E) pure water

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following liquids contains the strongest acid?
A) 0.1 M HA, pH = 6.85
B) 0.1 M HD, pH = 7.22
C) 0.1 M HE, pH = 8.34
D) 0.1 M HJ, pH = 11.88
E) pure water
A) 0.1 M HA, pH = 6.85
B) 0.1 M HD, pH = 7.22
C) 0.1 M HE, pH = 8.34
D) 0.1 M HJ, pH = 11.88
E) pure water

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
26
What is the pH of a 0.20 M HCl solution?
A) < 0
B) 0.70
C) 1.61
D) 12.39
E) 13.30
A) < 0
B) 0.70
C) 1.61
D) 12.39
E) 13.30

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
27
What is the [OH¯] for a solution at 25 C that has pH = 4.29?
A) 1.4 *10¯2 M
B) 5.l * 10¯5 M
C) 1.9 * 10¯10 M
D) 7.3 * 10¯13 M
E) 9.71 M
A) 1.4 *10¯2 M
B) 5.l * 10¯5 M
C) 1.9 * 10¯10 M
D) 7.3 * 10¯13 M
E) 9.71 M

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
28
What is the pH of a 0.050 M LiOH solution?
A) < 1.0
B) 1.30
C) 3.00
D) 11.00
E) 12.70
A) < 1.0
B) 1.30
C) 3.00
D) 11.00
E) 12.70

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
29
Select the correct relationship among the concentrations of species present in a 1.0 M aqueous solution of the weak acid represented by HA.
A) [H2O] > [A¯] ~ [H3O+] > [HA] > [OH¯]
B) [H2O] > [HA] > [A¯] > [H3O+] > [OH¯]
C) [HA] > [H2O] > [A¯] > [H3O+] > [OH¯]
D) [H2O] > [HA] > [A¯] ~ [H3O+] > [OH¯]
E) [HA] > [H2O] > [A¯] ~ [H3O+] > [OH¯]
A) [H2O] > [A¯] ~ [H3O+] > [HA] > [OH¯]
B) [H2O] > [HA] > [A¯] > [H3O+] > [OH¯]
C) [HA] > [H2O] > [A¯] > [H3O+] > [OH¯]
D) [H2O] > [HA] > [A¯] ~ [H3O+] > [OH¯]
E) [HA] > [H2O] > [A¯] ~ [H3O+] > [OH¯]

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
30
An aqueous solution is considered to be acidic if
A) the hydroxide ion concentration is 10¯6 M.
B) the hydrogen ion concentration is 10¯8 M.
C) the hydroxide and hydrogen ion concentrations are equal.
D) the hydroxide ion concentration is greater than the hydrogen ion concentration.
E) the hydroxide ion concentration is 10¯10 M.
A) the hydroxide ion concentration is 10¯6 M.
B) the hydrogen ion concentration is 10¯8 M.
C) the hydroxide and hydrogen ion concentrations are equal.
D) the hydroxide ion concentration is greater than the hydrogen ion concentration.
E) the hydroxide ion concentration is 10¯10 M.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
31
What is the [H3O+] for a solution at 25 C that has pOH = 5.640?
A) 2.34 *10¯4 M
B) 2.29 * 10¯6 M
C) 4.37 * 10¯9 M
D) 4.27 * 10¯11 M
E) 8.360 M
A) 2.34 *10¯4 M
B) 2.29 * 10¯6 M
C) 4.37 * 10¯9 M
D) 4.27 * 10¯11 M
E) 8.360 M

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
32
What is the [OH¯] for a solution at 25 C that has [H3O+] = 2.35 * 10¯3 M?
A) 4.26 * 10¯5 M
B) 2.35 *10¯11 M
C) 4.26 * 10¯12 M
D) 2.35 *10¯17 M
E) None of these choices is correct.
A) 4.26 * 10¯5 M
B) 2.35 *10¯11 M
C) 4.26 * 10¯12 M
D) 2.35 *10¯17 M
E) None of these choices is correct.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
33
What is the pH of a 0.75 M HNO3 solution?
A) 0.12
B) 0.29
C) 0.63
D) 0.82
E) > 1.0
A) 0.12
B) 0.29
C) 0.63
D) 0.82
E) > 1.0

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
34
What is the pH of a 0.00200 M HClO4 solution?
A) 0.995
B) 1.378
C) 2.699
D) 6.215
E) None of these choices is correct.
A) 0.995
B) 1.378
C) 2.699
D) 6.215
E) None of these choices is correct.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
35
What is the pH of a 0.0125 M NaOH solution?
A) 0.972
B) 1.903
C) 12.097
D) 13.028
E) None of these choices is correct.
A) 0.972
B) 1.903
C) 12.097
D) 13.028
E) None of these choices is correct.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
36
What is the pOH of a 0.0085 M KOH solution?
A) 2.07
B) 4.77
C) 9.23
D) 11.93
E) None of these choices is correct.
A) 2.07
B) 4.77
C) 9.23
D) 11.93
E) None of these choices is correct.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
37
What is the pH of a 0.050 M HBr solution?
A) 0.89
B) 1.12
C) 1.30
D) 3.00
E) None of these choices is correct.
A) 0.89
B) 1.12
C) 1.30
D) 3.00
E) None of these choices is correct.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
38
The hydrated Al3+ ion, Al(H2O)63+, is a weak acid in water. What are the products of its reaction with H2O?
Al(H2O)63+(aq) + H2O(l) ?
A) Al(H2O)5OH2+(aq) + H3O+(aq)
B) Al(H2O)6H4+(aq) + OH¯( aq)
C) Al(H2O)53+(aq) + 2H2O(l)
D) Al(H2O)6OH2+(aq) + H3O+( aq)
E) Al(H2O)62+(aq) + H3O+( aq)
Al(H2O)63+(aq) + H2O(l) ?
A) Al(H2O)5OH2+(aq) + H3O+(aq)
B) Al(H2O)6H4+(aq) + OH¯( aq)
C) Al(H2O)53+(aq) + 2H2O(l)
D) Al(H2O)6OH2+(aq) + H3O+( aq)
E) Al(H2O)62+(aq) + H3O+( aq)

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
39
What is the pOH of a 0.0250 M HI solution?
A) 0.944
B) 1.602
C) 12.398
D) 13.056
E) None of these choices is correct.
A) 0.944
B) 1.602
C) 12.398
D) 13.056
E) None of these choices is correct.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
40
When 14.7 mL of aqueous HBr (a strong acid) was added to water, 0.482 L of a solution with a pH of 4.23 was produced. What was the molarity of the original HBr solution?
A) 1.9 * 10¯3 M
B) 140 M
C) 0.288 M
D) 0.13 M
E) None of these choices is correct.
A) 1.9 * 10¯3 M
B) 140 M
C) 0.288 M
D) 0.13 M
E) None of these choices is correct.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
41
Picric acid has been used in the leather industry and in etching copper. However, its laboratory use has been restricted because it dehydrates on standing and can become shock sensitive. It has an acid dissociation constant of 0.42. What is the [H3O+] for a 0.20 M solution of picric acid?
A) 0.022 M
B) 0.052 M
C) 0.15 M
D) 0.29 M
E) None of these choices is correct.
A) 0.022 M
B) 0.052 M
C) 0.15 M
D) 0.29 M
E) None of these choices is correct.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
42
Which one of the following pairs is not a conjugate acid-base pair?
A) H2O, OH¯
B) H2O2, HO2¯
C) OH¯, O2¯
D) H2PO4¯, HPO42¯
E) HCl, H+
A) H2O, OH¯
B) H2O2, HO2¯
C) OH¯, O2¯
D) H2PO4¯, HPO42¯
E) HCl, H+

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
43
What is the value of Kb for the formate anion, HCOO¯? Ka(HCOOH) = 2.1 * 10¯4
A) -2.1 * 10¯4
B) 2.1 * 10¯4
C) 6.9 * 10¯6
D) 4.8 * 10¯11
E) 2.1 *10¯18
A) -2.1 * 10¯4
B) 2.1 * 10¯4
C) 6.9 * 10¯6
D) 4.8 * 10¯11
E) 2.1 *10¯18

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
44
According to Brønsted and Lowry, which one of the following is not a conjugate acid-base pair?
A) H3O+, OH¯
B) CH3OH2+, CH3OH
C) HI, I¯
D) HSO4¯, SO42¯
E) H2, H¯
A) H3O+, OH¯
B) CH3OH2+, CH3OH
C) HI, I¯
D) HSO4¯, SO42¯
E) H2, H¯

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
45
What is the pH of a 0.050 M triethylamine, (C2H5)3N, solution? Kb for triethylamine is 5.3 *10¯4.
A) 11.69
B) 8.68
C) 5.32
D) 2.31
E) < 2.0
A) 11.69
B) 8.68
C) 5.32
D) 2.31
E) < 2.0

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
46
A 0.15 M solution of chloroacetic acid has a pH of 1.86. What is the value of Ka for this acid?
A) 7.2 * 101
B) 0.16
C) 0.099
D) 0.0014
E) 0.00027
A) 7.2 * 101
B) 0.16
C) 0.099
D) 0.0014
E) 0.00027

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
47
Formic acid, which is a component of insect venom, has a Ka = 1.8 * 10¯4. What is the [H3O+] in a solution that is initially 0.10 M formic acid, HCOOH?
A) 4.2 * 10¯3 M
B) 8.4 * 10¯3 M
C) 1.8 * 10¯4 M
D) 1.8 * 10¯5 M
E) 1.8 * 10¯6 M
A) 4.2 * 10¯3 M
B) 8.4 * 10¯3 M
C) 1.8 * 10¯4 M
D) 1.8 * 10¯5 M
E) 1.8 * 10¯6 M

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
48
Select the pair of substances in which an acid is listed followed by its conjugate base.
A) H+, HCl
B) NH3, NH4+
C) HPO42¯, H2PO4¯
D) HCO3¯, CO32¯
E) CH3COOH, CH3COOH2+
A) H+, HCl
B) NH3, NH4+
C) HPO42¯, H2PO4¯
D) HCO3¯, CO32¯
E) CH3COOH, CH3COOH2+

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
49
Phosphoric acid, H3PO4, is a triprotic acid, for which Ka1 = 7.2 * 10¯3, Ka2 = 6.3 * 10¯8 and Ka3 = 4.2 * 10¯13. What is the value of Kb for the hydrogen phosphate anion, HPO42¯?
A) 6.3 * 10¯8
B) 4.2 * 10¯13
C) 1.4 * 10¯12
D) 1.6 * 10¯7
E) 2.4 * 10¯2
A) 6.3 * 10¯8
B) 4.2 * 10¯13
C) 1.4 * 10¯12
D) 1.6 * 10¯7
E) 2.4 * 10¯2

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
50
Lactic acid has a pKa of 3.08. What is the approximate degree of dissociation of a 0.35 M solution of lactic acid?
A) 1.1%
B) 2.2%
C) 4.8%
D) 14%
E) None of these choices is correct.
A) 1.1%
B) 2.2%
C) 4.8%
D) 14%
E) None of these choices is correct.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
51
Aqueous solutions of phosphoric acid and sodium nitrite are combined, and the following equilibrium is established. 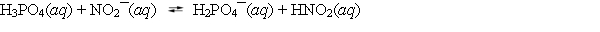 The equilibrium constant Kc for this reaction is greater than one. Based on this information, which of the following statements is correct?
The equilibrium constant Kc for this reaction is greater than one. Based on this information, which of the following statements is correct?
A) Phosphoric acid is a weaker acid than nitrous acid.
B) Nitrous acid is a weaker acid than water.
C) The nitrite anion is a weaker base than the dihydrogen phosphate anion.
D) The dihydrogen phosphate anion is a stronger acid than nitrous acid.
E) Phosphoric acid is a stronger acid than nitrous acid.
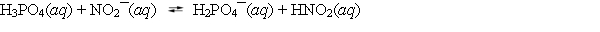 The equilibrium constant Kc for this reaction is greater than one. Based on this information, which of the following statements is correct?
The equilibrium constant Kc for this reaction is greater than one. Based on this information, which of the following statements is correct?A) Phosphoric acid is a weaker acid than nitrous acid.
B) Nitrous acid is a weaker acid than water.
C) The nitrite anion is a weaker base than the dihydrogen phosphate anion.
D) The dihydrogen phosphate anion is a stronger acid than nitrous acid.
E) Phosphoric acid is a stronger acid than nitrous acid.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
52
Arsenic acid, H3AsO4, is used industrially to manufacture insecticides. Arsenic acid is a polyprotic acid with K1 = 2.5 * 10¯4, K2 = 5.6 * 10¯8, and K3 = 3 *10¯13. What is the concentration of the HAsO42¯ in a solution whose initial arsenic acid concentration was 0.35 M ?
A) 9.4 *10¯3 M
B) 2.5 * 10¯4 M
C) 8.8 *10¯5 M
D) 5.6 * 10¯8 M
E) None of these choices is correct.
A) 9.4 *10¯3 M
B) 2.5 * 10¯4 M
C) 8.8 *10¯5 M
D) 5.6 * 10¯8 M
E) None of these choices is correct.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
53
Butyric acid is responsible for the odor in rancid butter. A solution of 0.25 M butyric acid has a pH of 2.71. What is the Ka for the acid?
A) 0.36
B) 2.4 *10¯2
C) 7.8 * 10¯3
D) 1.5 * 10¯5
E) None of these choices is correct.
A) 0.36
B) 2.4 *10¯2
C) 7.8 * 10¯3
D) 1.5 * 10¯5
E) None of these choices is correct.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
54
A 0.050 M solution of the weak acid HA has [H3O+] = 3.77 * 10¯4 M. What is the Ka for the acid?
A) 7.5 * 10¯3 M
B) 2.8 *10¯6 M
C) 7.0 * 10¯7 M
D) 7.0 * 10¯8 M
E) 2.6 *10¯11 M
A) 7.5 * 10¯3 M
B) 2.8 *10¯6 M
C) 7.0 * 10¯7 M
D) 7.0 * 10¯8 M
E) 2.6 *10¯11 M

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
55
A student adds 0.1 mol of oxalic acid and 0.1 mol of sodium dihydrogen phosphate to enough water to make 1.0 L of solution. The following equilibrium is established with the concentrations of the products greater than the concentrations of the reactants. Which of the statements about the equilibrium system is correct? 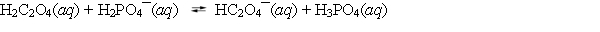
A) Oxalic acid is a weaker acid than phosphoric acid.
B) The hydrogen oxalate anion, HC2O4¯, is a stronger base than the dihydrogen phosphate anion, H2PO4¯.
C) Phosphoric acid is a weaker acid than oxalic acid.
D) The dihydrogen phosphate anion, H2PO4¯, is a stronger acid than oxalic acid.
E) Water is a stronger acid than either oxalic or phosphoric acids.
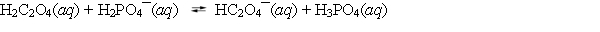
A) Oxalic acid is a weaker acid than phosphoric acid.
B) The hydrogen oxalate anion, HC2O4¯, is a stronger base than the dihydrogen phosphate anion, H2PO4¯.
C) Phosphoric acid is a weaker acid than oxalic acid.
D) The dihydrogen phosphate anion, H2PO4¯, is a stronger acid than oxalic acid.
E) Water is a stronger acid than either oxalic or phosphoric acids.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
56
Farmers who raise cotton once used arsenic acid, H3AsO4, as a defoliant at harvest time. Arsenic acid is a polyprotic acid with K1 = 2.5 *10¯4, K2 = 5.6 * 10¯8, and K3 = 3 *10¯13. What is the pH of a 0.500 M solution of arsenic acid?
A) 0.85
B) 1.96
C) 3.90
D) 4.51
E) None of these choices is correct.
A) 0.85
B) 1.96
C) 3.90
D) 4.51
E) None of these choices is correct.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
57
Select the pair of substances which is not a conjugate acid-base pair.
A) H3O+, H2O
B) HNO2, NO2¯
C) H2SO4, HSO4¯
D) H2S, S2¯
E) NH3, NH2¯
A) H3O+, H2O
B) HNO2, NO2¯
C) H2SO4, HSO4¯
D) H2S, S2¯
E) NH3, NH2¯

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
58
The acid dissociation constant Ka equals 1.26 *10¯2 for HSO4¯ and is 5.6 *10¯10 for NH4+. Which statement about the following equilibrium is correct? 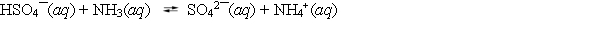
A) The reactants will be favored because ammonia is a stronger base than the sulfate anion.
B) The products will be favored because the hydrogen sulfate ion is a stronger acid than the ammonium ion.
C) Neither reactants nor products will be favored because all of the species are weak acids or bases.
D) The initial concentrations of the hydrogen sulfate ion and ammonia must be known before any prediction can be made.
E) This reaction is impossible to predict, since the strong acid and the weak base appear on the same side of the equation.
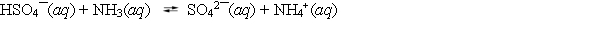
A) The reactants will be favored because ammonia is a stronger base than the sulfate anion.
B) The products will be favored because the hydrogen sulfate ion is a stronger acid than the ammonium ion.
C) Neither reactants nor products will be favored because all of the species are weak acids or bases.
D) The initial concentrations of the hydrogen sulfate ion and ammonia must be known before any prediction can be made.
E) This reaction is impossible to predict, since the strong acid and the weak base appear on the same side of the equation.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
59
Hydroxylamine, HONH2, readily forms salts such as hydroxylamine hydrochloride which are used as antioxidants in soaps. Hydroxylamine has Kb of 9.1 * 10¯9. What is the pH of a 0.025 M HONH2 solution?
A) 2.90
B) 4.82
C) 9.18
D) 9.91
E) 11.10
A) 2.90
B) 4.82
C) 9.18
D) 9.91
E) 11.10

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
60
A 1.25 M solution of the weak acid HA is 9.2% dissociated. What is the pH of the solution?
A) 0.64
B) 0.94
C) 1.13
D) 2.16
E) None of these choices is correct.
A) 0.64
B) 0.94
C) 1.13
D) 2.16
E) None of these choices is correct.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
61
A solution is prepared by adding 0.10 mol of lithium nitrate, LiNO3, to 1.00 L of water. Which statement about the solution is correct?
A) The solution is basic.
B) The solution is neutral.
C) The solution is weakly acidic.
D) The solution is strongly acidic.
E) The values for Ka and Kb for the species in solution must be known before a prediction can be made.
A) The solution is basic.
B) The solution is neutral.
C) The solution is weakly acidic.
D) The solution is strongly acidic.
E) The values for Ka and Kb for the species in solution must be known before a prediction can be made.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following is considered a Lewis acid?
A) CH3NH2
B) BCl3
C) F¯
D) BF4¯
E) CH4
A) CH3NH2
B) BCl3
C) F¯
D) BF4¯
E) CH4

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following aqueous liquids is the most acidic?
A) 0.1 M Al(NO3)3, Ka = 1 *10¯5
B) 0.1 M Be(NO3)2, Ka = 4 *10¯6
C) 0.1 M Pb(NO3)2, Ka = 3 *10¯8
D) 0.1 M Ni(NO3)2, Ka = 1 * 10¯10
E) pure water
A) 0.1 M Al(NO3)3, Ka = 1 *10¯5
B) 0.1 M Be(NO3)2, Ka = 4 *10¯6
C) 0.1 M Pb(NO3)2, Ka = 3 *10¯8
D) 0.1 M Ni(NO3)2, Ka = 1 * 10¯10
E) pure water

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
64
A solution is prepared by adding 0.10 mol of iron(III) nitrate, Fe(NO3)3, to 1.00 L of water. Which statement about the solution is correct?
A) The solution is basic.
B) The solution is neutral.
C) The solution is acidic.
D) The value of Ka for the species in solution must be known before a prediction can be made.
E) The value of Kb for the species in solution must be known before a prediction can be made.
A) The solution is basic.
B) The solution is neutral.
C) The solution is acidic.
D) The value of Ka for the species in solution must be known before a prediction can be made.
E) The value of Kb for the species in solution must be known before a prediction can be made.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
65
What is the value of Ka for the methylammonium ion, CH3NH3+? Kb(CH3NH2) = 4.4 *10¯4
A) 4.4 * 10¯4
B) 4.8 * 10¯6
C) 4.4 * 10¯10
D) 2.3 * 10¯11
E) 4.4 * 10¯18
A) 4.4 * 10¯4
B) 4.8 * 10¯6
C) 4.4 * 10¯10
D) 2.3 * 10¯11
E) 4.4 * 10¯18

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
66
What is the value of Kb for the cyanide anion, CN¯? Ka(HCN) = 6.2 * 10¯10
A) 1.6 * 10¯4
B) 1.6 * 10¯5
C) 3.8 *10¯4
D) 3.8 * 10¯5
E) 6.2 * 104
A) 1.6 * 10¯4
B) 1.6 * 10¯5
C) 3.8 *10¯4
D) 3.8 * 10¯5
E) 6.2 * 104

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following is a Lewis base?
A) BCl3
B) Cu2+
C) Cl¯
D) Mn2+
E) NH4+
A) BCl3
B) Cu2+
C) Cl¯
D) Mn2+
E) NH4+

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
68
Ammonium chloride is used as an electrolyte in dry cells. Which of the following statements about a 0.10 M solution of NH4Cl, is correct?
A) The solution is weakly basic.
B) The solution is strongly basic.
C) The solution is neutral.
D) The solution is acidic.
E) The values for Ka and Kb for the species in solution must be known before a prediction can be made.
A) The solution is weakly basic.
B) The solution is strongly basic.
C) The solution is neutral.
D) The solution is acidic.
E) The values for Ka and Kb for the species in solution must be known before a prediction can be made.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
69
A solution is prepared by adding 0.10 mol of potassium chloride, KCl, to 1.00 L of water. Which statement about the solution is correct?
A) The solution is basic.
B) The solution is neutral.
C) The solution is acidic.
D) One needs to know the temperature before any of the above predictions can be made.
E) The values for Ka and Kb for the species in solution must be known before a prediction can be made.
A) The solution is basic.
B) The solution is neutral.
C) The solution is acidic.
D) One needs to know the temperature before any of the above predictions can be made.
E) The values for Ka and Kb for the species in solution must be known before a prediction can be made.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
70
A solution is prepared by adding 0.10 mol of potassium acetate, KCH3COO, to 1.00 L of water. Which statement about the solution is correct?
A) The solution is basic.
B) The solution is neutral.
C) The solution is acidic.
D) The concentrations of potassium ions and acetate ions will be identical.
E) The concentration of acetate ions will be greater than the concentration of potassium ions.
A) The solution is basic.
B) The solution is neutral.
C) The solution is acidic.
D) The concentrations of potassium ions and acetate ions will be identical.
E) The concentration of acetate ions will be greater than the concentration of potassium ions.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
71
Which one of the following substances will give an aqueous solution of pH closest to 7?
A) KNO3
B) CO2
C) NH4I
D) NH3
E) CH3NH2
A) KNO3
B) CO2
C) NH4I
D) NH3
E) CH3NH2

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
72
A solution is prepared by adding 0.10 mol of sodium fluoride, NaF, to 1.00 L of water. Which statement about the solution is correct?
A) The solution is basic.
B) The solution is neutral.
C) The solution is acidic.
D) The concentrations of fluoride ions and sodium ions will be identical.
E) The concentration of fluoride ions will be greater than the concentration of sodium ions.
A) The solution is basic.
B) The solution is neutral.
C) The solution is acidic.
D) The concentrations of fluoride ions and sodium ions will be identical.
E) The concentration of fluoride ions will be greater than the concentration of sodium ions.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
73
What is the pH of a 0.0100 M sodium benzoate solution?
Kb (C7H5O2¯) = 1.5 * 10¯10
A) 0.38
B) 5.91
C) 8.09
D) 9.82
E) 13.62
Kb (C7H5O2¯) = 1.5 * 10¯10
A) 0.38
B) 5.91
C) 8.09
D) 9.82
E) 13.62

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
74
A solution is prepared by adding 0.10 mol of sodium sulfide, Na2S, to 1.00 L of water. Which statement about the solution is correct?
A) The solution is basic.
B) The solution is neutral.
C) The solution is acidic.
D) The concentration of sodium ions and sulfide ions will be identical.
E) The concentration of sulfide ions will be greater than the concentration of sodium ions.
A) The solution is basic.
B) The solution is neutral.
C) The solution is acidic.
D) The concentration of sodium ions and sulfide ions will be identical.
E) The concentration of sulfide ions will be greater than the concentration of sodium ions.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
75
Iodine trichloride, ICl3, will react with a chloride ion to form ICl4¯. Which species, if any, acts as a Lewis acid in this reaction?
A) ICl4¯
B) ICl3
C) Cl¯
D) the solvent
E) None of the species acts as a Lewis acid in this reaction.
A) ICl4¯
B) ICl3
C) Cl¯
D) the solvent
E) None of the species acts as a Lewis acid in this reaction.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
76
Calcium oxide, CaO, also known as quick lime, will react with carbon dioxide to form calcium carbonate, CaCO3. Which species, if any, acts as a Lewis acid in the reaction?
A) Ca2+
B) O2¯
C) CO2
D) CaCO3
E) None of the species acts as a Lewis acid in this reaction.
A) Ca2+
B) O2¯
C) CO2
D) CaCO3
E) None of the species acts as a Lewis acid in this reaction.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
77
What is the pH of a 0.010 M triethanolammonium chloride, (HOC2H2)3NHCl, solution?
Kb, ((HOC2H2)3N) = 5.9 *10¯7
A) 2.75
B) 4.89
C) 9.11
D) 11.25
E) None of these choices is correct.
Kb, ((HOC2H2)3N) = 5.9 *10¯7
A) 2.75
B) 4.89
C) 9.11
D) 11.25
E) None of these choices is correct.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
78
(p. 812 and other sections) Which of the following aqueous liquids will have the highest pH?
A) 0.1 M CH3COOH, pKa = 4.7
B) 0.1 M CuCl2, pKa = 7.5
C) 0.1 M H3C6H5O7, pKa = 3.1
D) 0.1 M ZnCl2, pKa = 9.0
E) pure water
A) 0.1 M CH3COOH, pKa = 4.7
B) 0.1 M CuCl2, pKa = 7.5
C) 0.1 M H3C6H5O7, pKa = 3.1
D) 0.1 M ZnCl2, pKa = 9.0
E) pure water

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
79
An aqueous solution is prepared by dissolving the salt formed by the neutralization of a weak acid by a weak base. Which statement about the solution is correct?
A) The solution is strongly basic.
B) The solution is weakly basic.
C) The solution is neutral.
D) The solution is acidic.
E) The values for Ka and Kb for the species in solution must be known before a prediction can be made.
A) The solution is strongly basic.
B) The solution is weakly basic.
C) The solution is neutral.
D) The solution is acidic.
E) The values for Ka and Kb for the species in solution must be known before a prediction can be made.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
80
Which one of the following substances will give an aqueous solution of pH < 7?
A) KI
B) NH4Br
C) Na2CO3
D) CH3COONa
E) CH3OH
A) KI
B) NH4Br
C) Na2CO3
D) CH3COONa
E) CH3OH

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck



