Deck 4: Chemical Bonding Understanding Climate Change
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/182
Play
Full screen (f)
Deck 4: Chemical Bonding Understanding Climate Change
1
Which statement below is FALSE?
A)Lattice energy reflects the amount of energy released when gas-phase ions combine to form a crystalline ionic lattice.
B)The energy of the ions in the solid ionic lattice is higher than that of the gas-phase ions.
C)Larger ions typically produce lattice energies that are smaller in magnitude.
D)As charge increases, the magnitude of the lattice energy increases.
E)The interaction of oppositely charged ions stabilizes the crystalline solid.
A)Lattice energy reflects the amount of energy released when gas-phase ions combine to form a crystalline ionic lattice.
B)The energy of the ions in the solid ionic lattice is higher than that of the gas-phase ions.
C)Larger ions typically produce lattice energies that are smaller in magnitude.
D)As charge increases, the magnitude of the lattice energy increases.
E)The interaction of oppositely charged ions stabilizes the crystalline solid.
The energy of the ions in the solid ionic lattice is higher than that of the gas-phase ions.
2
Coulomb's law states that the interaction energy between ions depends
A)only on the ionic charges.
B)only on the distance between the ions.
C)directly on both the ionic charges and the distance between the ions.
D)on the temperature.
E)directly on the ionic charges and inversely on the distance between the ions.
A)only on the ionic charges.
B)only on the distance between the ions.
C)directly on both the ionic charges and the distance between the ions.
D)on the temperature.
E)directly on the ionic charges and inversely on the distance between the ions.
directly on the ionic charges and inversely on the distance between the ions.
3
Which type of bonding involves the sharing of valence electrons by two atoms?
A)covalent
B)ionic
C)polar ionic
D)intramolecular
E)metallic
A)covalent
B)ionic
C)polar ionic
D)intramolecular
E)metallic
covalent
4
A covalent bond results when
A)electrons are transferred from one atom to another atom.
B)atoms pool their electrons to form a "sea" of electrons.
C)atoms have outer electrons with the same principal quantum number.
D)electrons are shared between a pair of atoms.
E)an atom has eight valence electrons.
A)electrons are transferred from one atom to another atom.
B)atoms pool their electrons to form a "sea" of electrons.
C)atoms have outer electrons with the same principal quantum number.
D)electrons are shared between a pair of atoms.
E)an atom has eight valence electrons.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
5
Which statement below regarding bonding is FALSE?
A)A single type of atom cannot form more than one kind of chemical bond.
B)The energy of separated atoms usually decreases when stable chemical compounds form.
C)Most compounds are more stable than the free elements that make them up.
D)The strength of a bond depends on the identities of the atoms involved.
E)Some atoms form different numbers of chemical bonds exhibit more than one valency).
A)A single type of atom cannot form more than one kind of chemical bond.
B)The energy of separated atoms usually decreases when stable chemical compounds form.
C)Most compounds are more stable than the free elements that make them up.
D)The strength of a bond depends on the identities of the atoms involved.
E)Some atoms form different numbers of chemical bonds exhibit more than one valency).

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
6
Calculate the electrostatic potential energy in joules of the interaction of one magnesium ion 86 pm radius) and one bromide ion 182 pm radius) in magnesium bromide.
A)(5.1 1019 J)
B)(1.0 1018 J)
C)(1.3 1018 J)
D)(8.6 1019 J )
E)(1.7 1018 J)
A)(5.1 1019 J)
B)(1.0 1018 J)
C)(1.3 1018 J)
D)(8.6 1019 J )
E)(1.7 1018 J)

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
7
Which statement below regarding covalent bonds is FALSE?
A)Electrons in a single covalent bond are mutually attracted to two nuclei.
B)The average bond length is the distance between the nuclei when the energy is at a minimum.
C)The energy of the interaction between two neutral atoms continuously decreases as their separation decreases.
D)The covalently bonded atoms are at a lower energy than the separated atoms.
E)Electron-nucleus attractions must overcome electron-electron and nucleus-nucleus repulsions.
A)Electrons in a single covalent bond are mutually attracted to two nuclei.
B)The average bond length is the distance between the nuclei when the energy is at a minimum.
C)The energy of the interaction between two neutral atoms continuously decreases as their separation decreases.
D)The covalently bonded atoms are at a lower energy than the separated atoms.
E)Electron-nucleus attractions must overcome electron-electron and nucleus-nucleus repulsions.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
8
Which statement below regarding ionic compounds is FALSE?
A)The net charge of an ionic compound is zero.
B)Oppositely charged ions collect together in part because isolated ions are not stable.
C)The energy associated with an ionic bond is a form of electrostatic potential energy.
D)Ions are held together by shared pairs of electrons.
E)Oppositely charged ions arrange themselves to minimize repulsions and maximize attractions.
A)The net charge of an ionic compound is zero.
B)Oppositely charged ions collect together in part because isolated ions are not stable.
C)The energy associated with an ionic bond is a form of electrostatic potential energy.
D)Ions are held together by shared pairs of electrons.
E)Oppositely charged ions arrange themselves to minimize repulsions and maximize attractions.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
9
When an ionic compound melts, the structure of the crystal lattice breaks down, and the ions must be able to slip past each other.Using electrostatic potential energy as a guide, identify the compound with the highest melting point.
A)NaF
B)KF
C)RbCl
D)ScF3
E)NaCl
A)NaF
B)KF
C)RbCl
D)ScF3
E)NaCl

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
10
Calculate the electrostatic potential energy in kilojoules per mole of the ionic bond between an aluminum ion (67.5 pm radius) and a nitride ion (182 pm radius) in aluminum nitride.
A)-5020 kJ/mol
B)-3350 kJ/mol
C)-1670 kJ/mol
D)-558 kJ/mol
E)-1050 kJ/mol
A)-5020 kJ/mol
B)-3350 kJ/mol
C)-1670 kJ/mol
D)-558 kJ/mol
E)-1050 kJ/mol

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
11
Bonding in metals is best described as
A)partial sharing of electrons between two atoms.
B)a network of covalent bonds.
C)a sea of pooled electrons.
D)highly directional and localized.
E)completely electrostatic.
A)partial sharing of electrons between two atoms.
B)a network of covalent bonds.
C)a sea of pooled electrons.
D)highly directional and localized.
E)completely electrostatic.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following statements regarding bond strength and length is true?
A)Energy is released when bonded atoms separate.
B)The length of a bond is the distance between two atoms when the potential energy of attraction between them is zero.
C)Bond strength increases as the distance between two atoms decreases.
D)When multiple pairs of electrons are shared between two atoms, bond strength decreases due to electron repulsions.
E)The length of a bond is generally inversely proportional to its strength.
A)Energy is released when bonded atoms separate.
B)The length of a bond is the distance between two atoms when the potential energy of attraction between them is zero.
C)Bond strength increases as the distance between two atoms decreases.
D)When multiple pairs of electrons are shared between two atoms, bond strength decreases due to electron repulsions.
E)The length of a bond is generally inversely proportional to its strength.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
13
When an ionic compound melts, the structure of the crystal lattice breaks down, and the ions must be able to slip past each other.Using electrostatic potential energy as a guide, which listing of ionic compounds is in order of increasing melting point?
A)AlF3 MgF2 NaF
B)MgF2 NaF AlF3
C)NaF MgF2 AlF3
D)AlF3 NaF MgF2
E)NaF AlF3 MgF2
A)AlF3 MgF2 NaF
B)MgF2 NaF AlF3
C)NaF MgF2 AlF3
D)AlF3 NaF MgF2
E)NaF AlF3 MgF2

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
14
Which ionic compound below will have the largest most negative) lattice energy?
A)NaF
B)NaCl
C)NaBr
D)CsF
E)CsCl
A)NaF
B)NaCl
C)NaBr
D)CsF
E)CsCl

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
15
Which compound below would you expect to have the smallest least negative) lattice energy?
A)MgBr2
B)CaBr2
C)CaO
D)BaBr2
E)BaO
A)MgBr2
B)CaBr2
C)CaO
D)BaBr2
E)BaO

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
16
Which one of the ionic compounds below would you expect to have the largest most negative) lattice energy?
A)MgO
B)CaO
C)SrO
D)BaO
E)BeO
A)MgO
B)CaO
C)SrO
D)BaO
E)BeO

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
17
Which statement below is FALSE?
A)Ionic bonds are typically formed when metal and nonmetal atoms interact.
B)The attraction between ions is primarily electrostatic.
C)Larger ions typically form stronger ionic bonds than smaller ions.
D)Ionic bond strength depends primarily on the radii and charges of the ions.
E)As ion charge increases, ionic bond strength increases.
A)Ionic bonds are typically formed when metal and nonmetal atoms interact.
B)The attraction between ions is primarily electrostatic.
C)Larger ions typically form stronger ionic bonds than smaller ions.
D)Ionic bond strength depends primarily on the radii and charges of the ions.
E)As ion charge increases, ionic bond strength increases.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
18
Which statement below requires the least energy to separate the ions?
A)CaF2
B)KF
C)NaF
D)MgF2
E)LiF
A)CaF2
B)KF
C)NaF
D)MgF2
E)LiF

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
19
Which ionic compound will require the greatest energy input to separate the ions?
A)MgI2
B)MgF2
C)MgCl2
D)MgBr2
E)NaCl
A)MgI2
B)MgF2
C)MgCl2
D)MgBr2
E)NaCl

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
20
Which compound below would you expect to have the smallest least negative) lattice energy?
A)MgCl2
B)CaCl2
C)AlCl3
D)MgI2
E)CaI2
A)MgCl2
B)CaCl2
C)AlCl3
D)MgI2
E)CaI2

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
21
Which compound below is ionic?
A)CF4
B)SO3
C)H2S
D)BaCl2
E)C12H22O11
A)CF4
B)SO3
C)H2S
D)BaCl2
E)C12H22O11

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
22
What is the formula for tinIV) oxide?
A)SnO
B)SnO2
C)Sn2O
D)SnO4
E)Sn2O2
A)SnO
B)SnO2
C)Sn2O
D)SnO4
E)Sn2O2

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
23
What is the formula for potassium oxide?
A)P2O
B)KO
C)K2O
D)PO2
E)KO2
A)P2O
B)KO
C)K2O
D)PO2
E)KO2

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
24
Which is the correct formula for hypochlorous acid?
A)HCl
B)HClO
C)HClO2
D)HClO3
E)HClO4
A)HCl
B)HClO
C)HClO2
D)HClO3
E)HClO4

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
25
You create a new bismuth compound.Which statement below is probably true about your compound?
A)It is probably ionic, and bismuth probably has a +3 charge.
B)It is probably ionic, and bismuth probably has a -3 charge.
C)The potential energy between the atoms (or ions) will be positive because bismuth is so large.
D)It is probably covalent because bismuth is a column VA nonmetal.
E)It is probably covalent because bismuth is a column VA metalloid.
A)It is probably ionic, and bismuth probably has a +3 charge.
B)It is probably ionic, and bismuth probably has a -3 charge.
C)The potential energy between the atoms (or ions) will be positive because bismuth is so large.
D)It is probably covalent because bismuth is a column VA nonmetal.
E)It is probably covalent because bismuth is a column VA metalloid.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
26
What is the correct name for CrCl3?
A)chromium(III) chloride
B)chromium trichloride
C)chromium(VI) trichloride
D)chromium(VI) chloride
E)chromium chloride
A)chromium(III) chloride
B)chromium trichloride
C)chromium(VI) trichloride
D)chromium(VI) chloride
E)chromium chloride

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
27
Which is the correct formula for sodium chlorite?
A)NaCl
B)NaClO
C)NaClO2
D)NaClO3
E)NaClO4
A)NaCl
B)NaClO
C)NaClO2
D)NaClO3
E)NaClO4

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
28
You create a new and unique ionic compound composed of +4 cations and -1 anions.Which of the following statements about your compound is FALSE?
A)The cations and anions must arrange themselves to minimize repulsions between ions with like charges.
B)There must be four times as many anions as cations in the crystal lattice.
C)Each cation must touch four anions.
D)The cation is probably significantly smaller than the anion, and this has an impact on the crystal structure.
E)The electrostatic potential energy is probably quite large and negative.
A)The cations and anions must arrange themselves to minimize repulsions between ions with like charges.
B)There must be four times as many anions as cations in the crystal lattice.
C)Each cation must touch four anions.
D)The cation is probably significantly smaller than the anion, and this has an impact on the crystal structure.
E)The electrostatic potential energy is probably quite large and negative.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
29
Metals are good conductors of electricity because
A)they are easily ionized.
B)their valence electrons are not localized.
C)they are easily reduced and oxidized.
D)they can be drawn into wires.
E)they are ductile.
A)they are easily ionized.
B)their valence electrons are not localized.
C)they are easily reduced and oxidized.
D)they can be drawn into wires.
E)they are ductile.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
30
Which compound below is molecular covalent)?
A)CuS
B)K2S
C)BaF2
D)SF4
E)AlCl3
A)CuS
B)K2S
C)BaF2
D)SF4
E)AlCl3

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
31
ManganeseIV) oxide is a brown insoluble solid often found as a product of the reactions of potassium permanganate.What is the formula of manganeseIV) oxide?
A)Mn4O
B)MnO4
C)MnO2
D)MnIV)O
E)Mn2O2
A)Mn4O
B)MnO4
C)MnO2
D)MnIV)O
E)Mn2O2

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
32
Aqua regia is a mixture of hydrochloric acid and nitric acid that is capable of dissolving gold.What are the formulas of these acids?
A)HClO, HNO4
B)HClO4, HNO3
C)HCl, HNO2
D)HCl, HNO3
E)HCl, HNO
A)HClO, HNO4
B)HClO4, HNO3
C)HCl, HNO2
D)HCl, HNO3
E)HCl, HNO

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
33
When H2S gas is dissolved in water, what is the name of the resulting binary acid?
A)hydrogen sulfuric acid
B)sulfuric acid
C)hydrosulfuric acid
D)bihydrosulfuric acid
E)hydrosulfic acid
A)hydrogen sulfuric acid
B)sulfuric acid
C)hydrosulfuric acid
D)bihydrosulfuric acid
E)hydrosulfic acid

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
34
Which is the correct formula for bromous acid?
A)HBr
B)HBrO4
C)HBrO3
D)HBrO2
E)HBrO
A)HBr
B)HBrO4
C)HBrO3
D)HBrO2
E)HBrO

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
35
Which is probably a good conductor of electricity?
A)S8
B)CuNi
C)ScI3
D)K2O
E)XeF4
A)S8
B)CuNi
C)ScI3
D)K2O
E)XeF4

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
36
Identify the binary compound that has ionic bonding.
A)N2O4
B)NF3
C)RbCl
D)CS2
E)H2S
A)N2O4
B)NF3
C)RbCl
D)CS2
E)H2S

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
37
Which compound is most likely to exhibit covalent bonding?
A)Fe2S3
B)MgO
C)CsBr
D)Cl2O
E)CuOH)2
A)Fe2S3
B)MgO
C)CsBr
D)Cl2O
E)CuOH)2

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
38
The proper name for Cr2S3 is
A)chromium(III) sulfide.
B)chromium(II) trisulfide.
C)chromium sulfide.
D)dichromium trisulfide.
E)chromium(III) trisulfide.
A)chromium(III) sulfide.
B)chromium(II) trisulfide.
C)chromium sulfide.
D)dichromium trisulfide.
E)chromium(III) trisulfide.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
39
What is the correct name of H2SO3?
A)sulfuric acid
B)sulfurous acid
C)hydrosulfuric acid
D)dihydrogen sulfurtrioxide acid
E)hydrosulfurous acid
A)sulfuric acid
B)sulfurous acid
C)hydrosulfuric acid
D)dihydrogen sulfurtrioxide acid
E)hydrosulfurous acid

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
40
Which ranks the compounds from lowest to highest lattice energy?
A)NaF MgF2 CaF2 KF
B)KF NaF CaF2 MgF2
C)MgF2 CaF2 NaF KF
D)CaF2 KF NaF MgF2
E)MgF2 CaF2 KF NaF
A)NaF MgF2 CaF2 KF
B)KF NaF CaF2 MgF2
C)MgF2 CaF2 NaF KF
D)CaF2 KF NaF MgF2
E)MgF2 CaF2 KF NaF

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
41
Active metals often form a protective oxide surface film that prevents further reaction of the metal with oxygen in the air.Which metal oxide formula below is NOT correct?
A)Al2O3 is aluminum oxide.
B)Fe2O3 is ironIII) oxide.
C)Na2O is sodium oxide.
D)MgO2 is magnesium oxide.
E)FeO is ironII) oxide.
A)Al2O3 is aluminum oxide.
B)Fe2O3 is ironIII) oxide.
C)Na2O is sodium oxide.
D)MgO2 is magnesium oxide.
E)FeO is ironII) oxide.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
42
Which statement below regarding Lewis dot symbols, Lewis structures, and the octet rule is FALSE?
A)The number of unpaired dots shows the typical bonding capacity of an atom.
B)Atoms tend to gain, lose, or share electrons in order to attain eight valence electrons.
C)There are some atoms that do not obey the octet rule.
D)A molecule cannot have an odd number of valence electrons.
E)Lewis dot structures can be written for atoms and for ions.
A)The number of unpaired dots shows the typical bonding capacity of an atom.
B)Atoms tend to gain, lose, or share electrons in order to attain eight valence electrons.
C)There are some atoms that do not obey the octet rule.
D)A molecule cannot have an odd number of valence electrons.
E)Lewis dot structures can be written for atoms and for ions.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
43
Which element below has five valence electrons?
A)Si
B)Se
C)S
D)I
E)Sb
A)Si
B)Se
C)S
D)I
E)Sb

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
44
How many valence electrons are in the Lewis structure of HNO2 ?
A)11
B)12
C)16
D)17
E)18
A)11
B)12
C)16
D)17
E)18

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
45
Buffer solutions that maintain certain levels of pH or acidity are widely used in biochemical experiments.One common buffer system uses sodium dihydrogenphosphate and sodium monohydrogenphosphate.What are the formulas of these two compounds?
A)NaHPO4 and NaHPO4)2
B)NaH2PO4 and Na2HPO4.
C)Na2H2PO4 and NaHPO4
D)NaPO4 and NaHPO4
E)Na2H2PO4 and NaHPO4.
A)NaHPO4 and NaHPO4)2
B)NaH2PO4 and Na2HPO4.
C)Na2H2PO4 and NaHPO4
D)NaPO4 and NaHPO4
E)Na2H2PO4 and NaHPO4.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
46
Zinc oxide is found in many ointments for the skin.What formula best describes this compound?
A)ZnO
B)Zn2O
C)ZnO2
D)Zn2O2
E)Zn2O3
A)ZnO
B)Zn2O
C)ZnO2
D)Zn2O2
E)Zn2O3

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
47
Which information below is NOT shown in Lewis structures of molecules?
A)how atoms are arranged in space
B)the number of bonding pairs of electrons between atoms
C)nonbonding electrons on atoms
D)the order in which atoms are linked
E)the number of valence electrons in the molecule
A)how atoms are arranged in space
B)the number of bonding pairs of electrons between atoms
C)nonbonding electrons on atoms
D)the order in which atoms are linked
E)the number of valence electrons in the molecule

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
48
Which Lewis symbol below is correct? 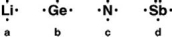
A)a
B)b
C)c
D)d
E)None of these is correct.
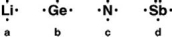
A)a
B)b
C)c
D)d
E)None of these is correct.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
49
Name the following oxides of nitrogen in this sequence: NO, N2O, NO2, N2O4.
A)nitrogen monoxide, dinitrogen monoxide, nitrogen dioxide, dinitrogen tetroxide
B)nitrox, dinitrox, nitridiox, dinitritriox
C)mononitrogen monoxide, dinitrogen monoxide, mononitrogen dioxide, dinitrogen tetraoxide
D)nitrogen oxide, nitrogen(II) oxide, nitrogen oxide(II), nitrogen(II) oxide(IV)
E)nitric oxide, nitrous oxide, nitrogen dioxide, nitrogen tetraoxide
A)nitrogen monoxide, dinitrogen monoxide, nitrogen dioxide, dinitrogen tetroxide
B)nitrox, dinitrox, nitridiox, dinitritriox
C)mononitrogen monoxide, dinitrogen monoxide, mononitrogen dioxide, dinitrogen tetraoxide
D)nitrogen oxide, nitrogen(II) oxide, nitrogen oxide(II), nitrogen(II) oxide(IV)
E)nitric oxide, nitrous oxide, nitrogen dioxide, nitrogen tetraoxide

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
50
Hydrogen can have at most electrons in its valence shell.
A)0
B)1
C)2
D)4
E)8
A)0
B)1
C)2
D)4
E)8

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
51
Which ion cannot be considered to have eight valence electrons?
A)Li+
B)Mg2+
C)N3-
D)F-
E)O2-
A)Li+
B)Mg2+
C)N3-
D)F-
E)O2-

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
52
How many valence electrons does arsenic have?
A)1
B)3
C)5
D)15
E)33
A)1
B)3
C)5
D)15
E)33

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
53
Sodium nitrite, which is used in meat processing, has been implicated as a possible health hazard because it can react with amines present in meat to form trace quantities of carcinogenic nitrosamines.What is the formula of sodium nitrite?
A)SNO
B)NaNO2
C)NaNO3
D)Na2NO4
E)Na2NO2
A)SNO
B)NaNO2
C)NaNO3
D)Na2NO4
E)Na2NO2

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
54
What is the proper name for Co2CO3)3?
A)cobalt tricarbonate
B)cobalt carbonate
C)cobalt oxycarbon
D)cobaltIII) carbonate
E)cobaltIII) carboxide
A)cobalt tricarbonate
B)cobalt carbonate
C)cobalt oxycarbon
D)cobaltIII) carbonate
E)cobaltIII) carboxide

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
55
How many valence electrons does S2- have?
A)4
B)6
C)8
D)14
E)16
A)4
B)6
C)8
D)14
E)16

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
56
Which ion below does NOT have either an empty valence shell or a complete valence-shell octet?
A)Al3
B)I
C)Ca2
D)PB2
E)Se2
A)Al3
B)I
C)Ca2
D)PB2
E)Se2

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
57
TiO2 is a white oxide used in paints.What is the proper name for TiO2?
A)titanium oxide
B)titaniumIV) oxide
C)titanic acid
D)titanium oxoate
E)titaniumIV) dioxide
A)titanium oxide
B)titaniumIV) oxide
C)titanic acid
D)titanium oxoate
E)titaniumIV) dioxide

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
58
Radium is often found in uranium ores and can be separated from solutions by precipitation as radium sulfate.What is the formula for radium sulfate?
A)RnSO4
B)RaSO4
C)Rn2SO3
D)Ra2SO4
E)RaSO4)2
A)RnSO4
B)RaSO4
C)Rn2SO3
D)Ra2SO4
E)RaSO4)2

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
59
On April 19, 1995, the Murrah Federal Building in Oklahoma City was destroyed-killing 168 people-by a simple but powerful bomb made from 4800 lb of ammonium nitrate.What is the formula for ammonium nitrate?
A)Am(NO3)2
B)Am(NO3)
C)NH4NO3
D)NH4(NO3)2
E)(NH4)2NO3
A)Am(NO3)2
B)Am(NO3)
C)NH4NO3
D)NH4(NO3)2
E)(NH4)2NO3

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
60
Which formula-name combinations below is NOT correct?
A)chlorine monoxide: ClO
B)chlorine dioxide: ClO2
C)dichlorine trioxide: Cl2O3
D)dichlorine monoxide: Cl2O
E)dichlorine heptoxide: Cl2O6
A)chlorine monoxide: ClO
B)chlorine dioxide: ClO2
C)dichlorine trioxide: Cl2O3
D)dichlorine monoxide: Cl2O
E)dichlorine heptoxide: Cl2O6

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
61
How many valence electrons are there in a correctly drawn Lewis structure for formamide, HCONH2?
A)12
B)14
C)16
D)18
E)20
A)12
B)14
C)16
D)18
E)20

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
62
How many covalent bonds are there in HS-?
A)1
B)2
C)3
D)0
E)4
A)1
B)2
C)3
D)0
E)4

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
63
Acetonitrile CH3CN) is an important industrial chemical.Among other things, it is used to make plastic moldings, which have multiple uses, from car parts to Lego bricks.Which statement below about acetonitrile is FALSE?
A)Acetonitrile has 16 valence electrons in its Lewis structure.
B)Acetonitrile has one triple bond.
C)Acetonitrile has one pair of nonbonding electrons.
D)All atoms satisfy the octet rule in acetonitrile.
E)One carbon atom and the nitrogen atom have nonzero formal charges.
A)Acetonitrile has 16 valence electrons in its Lewis structure.
B)Acetonitrile has one triple bond.
C)Acetonitrile has one pair of nonbonding electrons.
D)All atoms satisfy the octet rule in acetonitrile.
E)One carbon atom and the nitrogen atom have nonzero formal charges.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
64
How many shared electrons are there in the Lewis structure of SbF3?
A)3
B)6
C)4
D)8
E)10
A)3
B)6
C)4
D)8
E)10

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
65
How many shared electron pairs are there in the Lewis structure of CH3SH?
A)3
B)4
C)5
D)6
E)8
A)3
B)4
C)5
D)6
E)8

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
66
Which molecule below contains the largest number of nonbonding electrons?
A)H2
B)CO
C)N2
D)NO
E)O2
A)H2
B)CO
C)N2
D)NO
E)O2

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
67
How many nonbonding electrons are there in the Lewis structure of SCO?
A)0
B)4
C)8
D)10
E)12
A)0
B)4
C)8
D)10
E)12

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
68
How many shared electron pairs are there in the Lewis structure of C2H4Cl2?
A)12
B)11
C)8
D)7
E)5
A)12
B)11
C)8
D)7
E)5

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
69
How many nonbonding electron pairs are there in the Lewis structure of CN-?
A)0
B)1
C)2
D)3
E)6
A)0
B)1
C)2
D)3
E)6

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
70
Vinegar is a solution of acetic acid (CH3COOH) and water.Which Lewis structure below for acetic acid is correct?
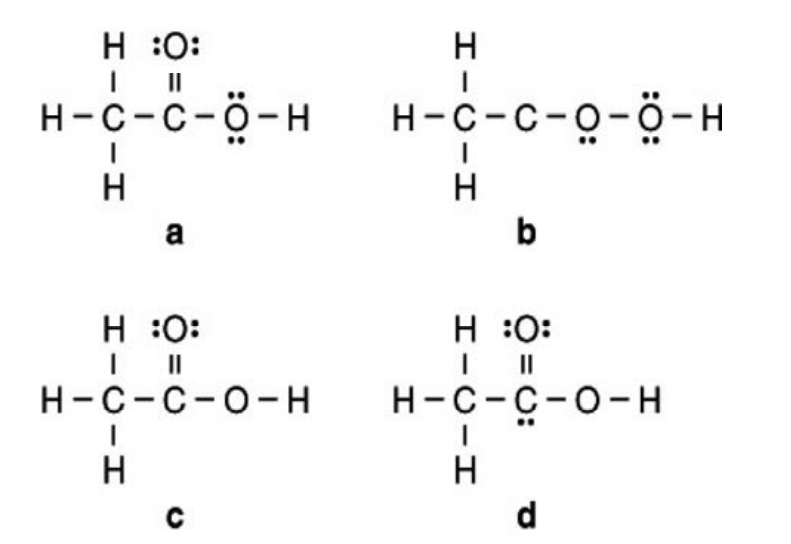
A)a
B)b
C)c
D)d
E)none of these

A)a
B)b
C)c
D)d
E)none of these

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
71
Which molecule below has four shared electrons?
A)O2
B)CO
C)HI
D)N2
E)Cl2
A)O2
B)CO
C)HI
D)N2
E)Cl2

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
72
How many covalent bonds are there in CN-?
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
73
Which statement below regarding the Lewis structure of atoms and ions is FALSE?
A)Charges on ions must be explicitly shown.
B)A magnesium ion with a +2 charge has no electron dots shown.
C)A neutral oxygen atom has two pairs of electron dots and two unpaired dots shown.
D)The chloride ion with a -1 charge has eight valence electrons dots shown.
E)The sodium ion has eight valence electron dots shown.
A)Charges on ions must be explicitly shown.
B)A magnesium ion with a +2 charge has no electron dots shown.
C)A neutral oxygen atom has two pairs of electron dots and two unpaired dots shown.
D)The chloride ion with a -1 charge has eight valence electrons dots shown.
E)The sodium ion has eight valence electron dots shown.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
74
Which molecule below contains a triple bond?
A)CO2
B)NCl3
C)C2H4
D)S2
E)N2
A)CO2
B)NCl3
C)C2H4
D)S2
E)N2

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
75
Which molecule below has only single bonds?
A)CO2
B)CO
C)HCN
D)OF2
E)SO2
A)CO2
B)CO
C)HCN
D)OF2
E)SO2

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
76
How many shared electrons are there in the Lewis structure of H2O2?
A)3
B)6
C)8
D)10
E)12
A)3
B)6
C)8
D)10
E)12

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
77
Which molecule below contains a double bond?
A)N2
B)CO
C)S2
D)CCl4
E)Cl2
A)N2
B)CO
C)S2
D)CCl4
E)Cl2

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
78
Which is the correct Lewis symbol for the oxide anion O2-)? 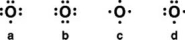
A)a
B)b
C)c
D)d
E)None of these is correct.
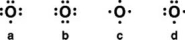
A)a
B)b
C)c
D)d
E)None of these is correct.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
79
How many shared electrons are there in the Lewis structure of HCN?
A)4
B)6
C)8
D)10
E)12
A)4
B)6
C)8
D)10
E)12

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
80
How many shared electron pairs are there in the Lewis structure of OF2?
A)6
B)4
C)3
D)2
E)1
A)6
B)4
C)3
D)2
E)1

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck



