Deck 12: Thermodynamics Why Chemical Reactions Happen
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/130
Play
Full screen (f)
Deck 12: Thermodynamics Why Chemical Reactions Happen
1
The molar entropy of ammonia, NH3g), at 25 C is 192.5 J/mol .K.Estimate the number of microstates accessible to a NH3 molecule at 25 C to two significant figures.
A)1.1 * 1010
B)1.4 *1025
C)6.8 * 1033
D)4.0 * 1083
E)23
A)1.1 * 1010
B)1.4 *1025
C)6.8 * 1033
D)4.0 * 1083
E)23
1.1 * 1010
2
Of the three modes of molecular motion-vibration, rotation, and translation-which requires the greatest amount of energy to cause an excitation from the ground state to the first excited state?
A)vibration
B)rotation
C)translation
D)They all require the same amount of energy.
E)none, as quantized energy states do not apply to these motions
A)vibration
B)rotation
C)translation
D)They all require the same amount of energy.
E)none, as quantized energy states do not apply to these motions
vibration
3
When the volume of a gas is increased, the energy separation between microstates
A)increases.
B)decreases.
C)remains unchanged.
D)becomes infinite.
E)disappears.
A)increases.
B)decreases.
C)remains unchanged.
D)becomes infinite.
E)disappears.
decreases.
4
Which of the following processes is NOT spontaneous?
A)Iron in moist air rusts.
B)Carbon dioxide and water react to form sugar and oxygen gas.
C)Liquid water in a freezer turns to ice.
D)A spark ignites a mixture of propane and air.
E)Baking soda sodium hydrogen carbonate) reacts with lemon juice dilute citric acid).
A)Iron in moist air rusts.
B)Carbon dioxide and water react to form sugar and oxygen gas.
C)Liquid water in a freezer turns to ice.
D)A spark ignites a mixture of propane and air.
E)Baking soda sodium hydrogen carbonate) reacts with lemon juice dilute citric acid).

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following is the best definition of a microstate as it pertains to entropy on the molecular level?
A)the state solid, liquid, or gas) of a small cluster of atoms or molecules
B)a property of a small cluster of atoms and molecules, such as temperature, that is independent of how the property was achieved
C)the set of vibrational, rotational, and translational energy levels in a system
D)a unique specification of the vibrational, rotational, and translational states of a single molecule in a system
E)a unique specification of the vibrational, rotational, and translational states of all the particles in a system
A)the state solid, liquid, or gas) of a small cluster of atoms or molecules
B)a property of a small cluster of atoms and molecules, such as temperature, that is independent of how the property was achieved
C)the set of vibrational, rotational, and translational energy levels in a system
D)a unique specification of the vibrational, rotational, and translational states of a single molecule in a system
E)a unique specification of the vibrational, rotational, and translational states of all the particles in a system

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following statements regarding spontaneous processes is NOT true?
A)Spontaneous processes are exothermic.
B)Spontaneous processes proceed without outside intervention once initiated.
C)Spontaneous reactions are not always rapid.
D)The motional freedom of particles tends to increase during spontaneous change.
E)Particles tend to become more spread out during spontaneous change.
A)Spontaneous processes are exothermic.
B)Spontaneous processes proceed without outside intervention once initiated.
C)Spontaneous reactions are not always rapid.
D)The motional freedom of particles tends to increase during spontaneous change.
E)Particles tend to become more spread out during spontaneous change.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
7
At 0 K, the entropy of a perfect crystal is
A)( 0)
B)(= 0)
C)( 0)
D)( 0, = 0, or 0, depending on the chemical structure of the crystal)
E)( 0 or = 0, depending on the chemical structure of the crystal)
A)( 0)
B)(= 0)
C)( 0)
D)( 0, = 0, or 0, depending on the chemical structure of the crystal)
E)( 0 or = 0, depending on the chemical structure of the crystal)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following must be true for the microstates of a system? I.The energy of each microstate equals the energy of the system.
II)The entropy of each microstate equals the entropy of the system.
III)The number of microstates equals the entropy of the system.
A)I only
B)II only
C)III only
D)I and III only
E)I, II, and III are all true
II)The entropy of each microstate equals the entropy of the system.
III)The number of microstates equals the entropy of the system.
A)I only
B)II only
C)III only
D)I and III only
E)I, II, and III are all true

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
9
At 25 C, a benzene molecule (C6H6) has approximately 1.143 * 1014 accessible microstates.Estimate the molar entropy of C6H6 at 25.0 C to two significant figures.
A)4.5 *10-22 J/mol . K
B)3.9 J/mol . K
C)14 J/mol . K
D)32 J/mol . K
E)2.7 * 102 J/mol . K
A)4.5 *10-22 J/mol . K
B)3.9 J/mol . K
C)14 J/mol . K
D)32 J/mol . K
E)2.7 * 102 J/mol . K

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
10
How many microstates are accessible to one mole of atoms in a perfect crystalline lattice at absolute zero?
A)0
B)1
C)1.381 * 10-23
D)52.64
E)6.022 * 1023
A)0
B)1
C)1.381 * 10-23
D)52.64
E)6.022 * 1023

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
11
Given one mole of each of the following, which of the following has the highest number of accessible microstates?
A)Ice, H2Os), at 273 K
B)Liquid water, H2Ol), at 373 K
C)Steam, H2Og), at 373 K
D)Propane, CH3CH2CH3g), at 373 K
E)Butane, CH3CH2CH2CH3g), at 373 K
A)Ice, H2Os), at 273 K
B)Liquid water, H2Ol), at 373 K
C)Steam, H2Og), at 373 K
D)Propane, CH3CH2CH3g), at 373 K
E)Butane, CH3CH2CH2CH3g), at 373 K

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following states of motion has the highest number of occupied excited states at room temperature-rotational, vibrational, or translational?
A)rotational
B)vibrational
C)translational
D)The number of occupied excited states is the same for the three modes of motion.
E)none of these, as excited states do not apply to these motions
A)rotational
B)vibrational
C)translational
D)The number of occupied excited states is the same for the three modes of motion.
E)none of these, as excited states do not apply to these motions

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following statements is a valid definition of entropy?
A)It is a measure of the average kinetic energies of the particles in a system.
B)It is a measure of the enthalpy changes during a spontaneous change.
C)It is a measure of how the temperature changes during a spontaneous change.
D)It is a measure of how randomly arranged the particles in a system are.
E)It is a measure of how dispersed the energy of a system is.
A)It is a measure of the average kinetic energies of the particles in a system.
B)It is a measure of the enthalpy changes during a spontaneous change.
C)It is a measure of how the temperature changes during a spontaneous change.
D)It is a measure of how randomly arranged the particles in a system are.
E)It is a measure of how dispersed the energy of a system is.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
14
What modes of motion does a molecule of chlorine have?
A)translational, rotational, and vibrational
B)kinetic energy and potential energy
C)microstate and macrostate
D)Boltzmann and non-Boltzmann
E)all of the above
A)translational, rotational, and vibrational
B)kinetic energy and potential energy
C)microstate and macrostate
D)Boltzmann and non-Boltzmann
E)all of the above

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
15
According to the second law of thermodynamics, the entropy change in an isolated system ( Ssys) during a spontaneous process must be
A)greater than zero.
B)less than zero.
C)equal to zero.
D)greater than or equal to zero.
E)greater than, less than, or equal to zero.
A)greater than zero.
B)less than zero.
C)equal to zero.
D)greater than or equal to zero.
E)greater than, less than, or equal to zero.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
16
An oxygen molecule at room temperature can access the largest number of accessible microstates associated with which mode of molecular motion?
A)vibrational
B)kinetic
C)translational
D)elastic
E)Boltzmann
A)vibrational
B)kinetic
C)translational
D)elastic
E)Boltzmann

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
17
Boltzmann derived the relationship S = k ln W, where W is the
A)number of microstates.
B)vibrational energy.
C)kinetic energy.
D)Wentworth factor.
E)potential energy.
A)number of microstates.
B)vibrational energy.
C)kinetic energy.
D)Wentworth factor.
E)potential energy.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is a driving force behind spontaneous reactions?
A)A solid is formed.
B)Heat is absorbed.
C)The reaction is rapid.
D)The motional freedom of the particles increases.
E)The enthalpy of the particles increases.
A)A solid is formed.
B)Heat is absorbed.
C)The reaction is rapid.
D)The motional freedom of the particles increases.
E)The enthalpy of the particles increases.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
19
The entropy change in a system ( Ssys) during a spontaneous process must be
A)greater than zero.
B)less than zero.
C)equal to zero.
D)greater than or equal to zero.
E)greater than, less than, or equal to zero.
A)greater than zero.
B)less than zero.
C)equal to zero.
D)greater than or equal to zero.
E)greater than, less than, or equal to zero.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
20
Perfect crystals of carbon monoxide (CO) are difficult to prepare because the very small dipole moment allows a few molecules to align in a pattern like CO OC CO instead of CO CO CO.If such crystals were cooled to 0 K, what would be the value of their absolute entropy?
A)( 0)
B)(= 0)
C)( 0)
D)( 0, = 0, or 0, depending on how carefully it was cooled)
E)( 0 or = 0, depending on how carefully it was cooled)
A)( 0)
B)(= 0)
C)( 0)
D)( 0, = 0, or 0, depending on how carefully it was cooled)
E)( 0 or = 0, depending on how carefully it was cooled)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
21
The absolute entropy of an NaCl crystal is
A)an intensive property and a state function.
B)an intensive property and a path function.
C)an extensive property and a state function.
D)an extensive property and a path function.
E)not appropriately described in terms of an intensive property, an extensive property, a state function, or a path function.
A)an intensive property and a state function.
B)an intensive property and a path function.
C)an extensive property and a state function.
D)an extensive property and a path function.
E)not appropriately described in terms of an intensive property, an extensive property, a state function, or a path function.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
22
Consider a closed container containing a 1.00 M solution of aqueous HCl at 298 K.Above the solution, air containing both water vapor and HCl gas at their equilibrium vapor pressures exerts a total pressure of 1.00 bar.Which of the following is/are in their thermodynamic standard state? I.the liquid water
II)the HCl solution
III)the water vapor
A)I only
B)II only
C)III only
D)I and II only
E)I, II, and III are all in their standard state.
II)the HCl solution
III)the water vapor
A)I only
B)II only
C)III only
D)I and II only
E)I, II, and III are all in their standard state.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following are listed in order of increasing standard molar entropy?
A)Au(s) Au(g) H2O(l) CH3OH(l) H2O(g)
B)Au(s) H2O(l) CH3OH(l) Au(g) H2O(g)
C)Au(s) H2O(l) CH3OH(l) H2O(g) Au(g)
D)H2O(l) CH3OH(l) Au(s) Au(g) H2O(g)
E)H2O(l) Au(s) CH3OH(l) H2O(g) Au(g)
A)Au(s) Au(g) H2O(l) CH3OH(l) H2O(g)
B)Au(s) H2O(l) CH3OH(l) Au(g) H2O(g)
C)Au(s) H2O(l) CH3OH(l) H2O(g) Au(g)
D)H2O(l) CH3OH(l) Au(s) Au(g) H2O(g)
E)H2O(l) Au(s) CH3OH(l) H2O(g) Au(g)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
24
In a spontaneous process, the entropy of the universe
A)always increases.
B)always decreases.
C)does not change.
D)may decrease if the entropy of the system decreases sufficiently.
E)may decrease if the entropy of the system increases sufficiently.
A)always increases.
B)always decreases.
C)does not change.
D)may decrease if the entropy of the system decreases sufficiently.
E)may decrease if the entropy of the system increases sufficiently.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following is in the correct order of standard state entropy? I.NaCl(aq) NaCl(s)
II.O2(g) O3(g)
III.Kr(g) Cl2(g)
A)I only
B)II only
C)III only
D)I and II only
E)II and III only
II.O2(g) O3(g)
III.Kr(g) Cl2(g)
A)I only
B)II only
C)III only
D)I and II only
E)II and III only

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
26
During a spontaneous chemical reaction, it is found that Ssys is less than 0.This means that
A)( Ssurr is less than 0 and its magnitude is less than Ssys.)
B)( Ssurr is less than 0 and its magnitude is greater than Ssys.)
C)( Ssurr is greater than 0 and its magnitude is less than Ssys.)
D)( Ssurr is greater than 0 and its magnitude is greater than Ssys.)
E)an error has been made, as Ssys is greater than 0 by necessity for a spontaneous process.
A)( Ssurr is less than 0 and its magnitude is less than Ssys.)
B)( Ssurr is less than 0 and its magnitude is greater than Ssys.)
C)( Ssurr is greater than 0 and its magnitude is less than Ssys.)
D)( Ssurr is greater than 0 and its magnitude is greater than Ssys.)
E)an error has been made, as Ssys is greater than 0 by necessity for a spontaneous process.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following statements regarding absolute and standard entropies is NOT true?
A)The standard entropy of a solution is the value of S when the solution is at 298 K and 1 M concentration.
B)The standard entropy of a gas is the value of S when the gas is at 298 K and 1 bar pressure.
C)The standard entropy of a pure solid or liquid is the value of S for its most stable allotrope at 298 K and 1 bar.
D)Elements in their standard states at 298 K and 1 bar have nonzero entropy values.
E)The entropy of a compound increases linearly with temperature.
A)The standard entropy of a solution is the value of S when the solution is at 298 K and 1 M concentration.
B)The standard entropy of a gas is the value of S when the gas is at 298 K and 1 bar pressure.
C)The standard entropy of a pure solid or liquid is the value of S for its most stable allotrope at 298 K and 1 bar.
D)Elements in their standard states at 298 K and 1 bar have nonzero entropy values.
E)The entropy of a compound increases linearly with temperature.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
28
The standard molar entropy of lead(II) bromide (PbBr2) is 161 J/mol .K.What is the entropy of 2.45 g of PbBr2?
A)(F+1.07 J/K)
B)(-1.07 J/K)
C)(+161 J/K)
D)(-161 J/K)
E)0 J/K
A)(F+1.07 J/K)
B)(-1.07 J/K)
C)(+161 J/K)
D)(-161 J/K)
E)0 J/K

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following is in the correct order of standard state entropy? I.Diamond graphite
II.Liquid water solid water
III.NH3 H2
A)I only
B)II only
C)III only
D)I and II only
E)I and III only
II.Liquid water solid water
III.NH3 H2
A)I only
B)II only
C)III only
D)I and II only
E)I and III only

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following processes is predicted to decrease the entropy of a system?
A)The number of particles increases.
B)Temperature increases.
C)Volume increases.
D)Pressure increases.
E)All of these increase the entropy of the system.
A)The number of particles increases.
B)Temperature increases.
C)Volume increases.
D)Pressure increases.
E)All of these increase the entropy of the system.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following will have the greatest standard molar entropy (S )?
A)Cl2(g)
B)Ne(g)
C)Na(s)
D)CH3OH(l)
E)Na2CO3(s)
A)Cl2(g)
B)Ne(g)
C)Na(s)
D)CH3OH(l)
E)Na2CO3(s)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
32
The molar entropies of carbon monoxide and carbon dioxide at 25 C are 197.7 and 213.8 J/mol . K, respectively.How many times more accessible microstates does CO2 have than CO at 25 C?
A)roughly 16 times more
B)roughly 1.1 times more
C)roughly 1.3 * 1011 times more
D)roughly 6.9 times more
E)roughly 1.9 times more
A)roughly 16 times more
B)roughly 1.1 times more
C)roughly 1.3 * 1011 times more
D)roughly 6.9 times more
E)roughly 1.9 times more

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
33
Indicate which of the following has the highest entropy at 298 K.
A)0.5 g of CO2
B)1 mol of CO2
C)2 kg of CO2
D)2 mol of CO2
E)All of the above have the same entropy at 298 K.
A)0.5 g of CO2
B)1 mol of CO2
C)2 kg of CO2
D)2 mol of CO2
E)All of the above have the same entropy at 298 K.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
34
According to the second law of thermodynamics, the change in the entropy of the universe ( Suniv) during a spontaneous reaction is
A)zero.
B)negative.
C)positive.
D)less than the change in entropy of the system ( Ssys).
E)greater than the change in entropy of the system ( Ssys).
A)zero.
B)negative.
C)positive.
D)less than the change in entropy of the system ( Ssys).
E)greater than the change in entropy of the system ( Ssys).

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
35
If a reaction is spontaneous under a given set of conditions,
A)( Ssurr is greater than 0 for the reverse reaction.)
B)the reverse reaction is nonspontaneous.
C)( Ssys for the reverse reaction is smaller than Ssys for the forward reaction.)
D)the reverse reaction is also spontaneous.
E)(Suniv is greater than 0 for the reverse reaction.)
A)( Ssurr is greater than 0 for the reverse reaction.)
B)the reverse reaction is nonspontaneous.
C)( Ssys for the reverse reaction is smaller than Ssys for the forward reaction.)
D)the reverse reaction is also spontaneous.
E)(Suniv is greater than 0 for the reverse reaction.)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
36
In a spontaneous process, which of the following always increases?
A)the entropy of the system
B)the entropy of the surroundings
C)the entropy of the universe
D)the entropy of the system and the universe
E)the entropy of the system, surroundings, and universe
A)the entropy of the system
B)the entropy of the surroundings
C)the entropy of the universe
D)the entropy of the system and the universe
E)the entropy of the system, surroundings, and universe

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
37
During which of the following processes does the entropy of the system decrease?
A)Salt crystals dissolve in water.
B)Air escapes from a hole in a balloon.
C)Iron and oxygen gas react to form rust.
D)Ice melts in your hand.
E)None of these decrease the entropy of the system.
A)Salt crystals dissolve in water.
B)Air escapes from a hole in a balloon.
C)Iron and oxygen gas react to form rust.
D)Ice melts in your hand.
E)None of these decrease the entropy of the system.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
38
During which of the following processes does the entropy of the system increase?
A)Water vapor condenses on a cool surface.
B)Helium gas escapes from a hole in a balloon.
C)Calcium carbonate stalactites form in a cave.
D)Water freezes in a freezer.
E)All of these increase entropy of the system.
A)Water vapor condenses on a cool surface.
B)Helium gas escapes from a hole in a balloon.
C)Calcium carbonate stalactites form in a cave.
D)Water freezes in a freezer.
E)All of these increase entropy of the system.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
39
When carbon monoxide forms a crystal, each molecule can be pictured as having two possible orientations, CO or OC, because the molecular dipole moment is quite small.Assuming two possible microstates for each CO molecule, calculate the value of S when one mole of CO forms a crystalline solid at 0 K.
A)0 J/mol . K
B)2.00 J/mol .Kl
C)5.76 J/mol . K
D)9.57 * 10-23 J/mol . K
E)The value of S is too small to calculate.
A)0 J/mol . K
B)2.00 J/mol .Kl
C)5.76 J/mol . K
D)9.57 * 10-23 J/mol . K
E)The value of S is too small to calculate.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
40
Indicate which of the following has the lowest standard molar entropy (S ).
A)CH4(g)
B)CH3CH2OH(l)
C)H2O(s)
D)Na(s)
E)He(g)
A)CH4(g)
B)CH3CH2OH(l)
C)H2O(s)
D)Na(s)
E)He(g)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
41
The enthalpy and entropy of vaporization of ammonia at its normal boiling point, -33.34 C, are approximately 23.35 kJ/mol and 97.4 J/mol . K, respectively.What would the entropy change of the universe be if one mole of ammonia vapor were to condense in a large room maintained at 30.0 C?
A)(+20.4 J/K)
B)(+77.0 J/K)
C)(-174.4 J/K)
D)(-20.4 J/K)
E)(+174.4 J/K)
A)(+20.4 J/K)
B)(+77.0 J/K)
C)(-174.4 J/K)
D)(-20.4 J/K)
E)(+174.4 J/K)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following must be true for a spontaneous exothermic process?
A)Only Ssys is less than 0.
B)Only Ssys is greater than 0.
C)Both Ssys is less than 0 and the magnitude of Ssys is less than the magnitude of Ssurr.
D)Both Ssys is less than 0 and the magnitude of Ssys is greater than the magnitude of Ssurr.
E)Either Ssys is greater than 0 or Ssys is less than 0 and the magnitude of Ssys is less than the magnitude of Ssurr.
A)Only Ssys is less than 0.
B)Only Ssys is greater than 0.
C)Both Ssys is less than 0 and the magnitude of Ssys is less than the magnitude of Ssurr.
D)Both Ssys is less than 0 and the magnitude of Ssys is greater than the magnitude of Ssurr.
E)Either Ssys is greater than 0 or Ssys is less than 0 and the magnitude of Ssys is less than the magnitude of Ssurr.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
43
The enthalpy of vaporization of ammonia at its normal boiling point,-33.34 C, is approximately 23.35 kJ/mol.What is the entropy change in the surroundings when one mole of liquid ammonia vaporizes at -33.34 C in a large room maintained at a temperature of 25.0 C?
A)(-2.40 *102 J/K)
B)(-78.3 J/K)
C)(-19.1 J/K)
D)(+78.3 J/K)
E)(+2.40 * 102 J/K)
A)(-2.40 *102 J/K)
B)(-78.3 J/K)
C)(-19.1 J/K)
D)(+78.3 J/K)
E)(+2.40 * 102 J/K)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following statements regarding reversible processes is NOT true?
A)Heat transfer between the system and surroundings usually occurs reversibly during ordinary chemical reactions.
B)The change in entropy of the universe is zero during a reversible process.
C)The entropy change in the surroundings is equal and opposite to that of the system during a reversible process.
D)Reversible change would require an infinite amount of time to complete.
E)Heat transfer between the system and surroundings during phase changes can be approximated as reversible.
A)Heat transfer between the system and surroundings usually occurs reversibly during ordinary chemical reactions.
B)The change in entropy of the universe is zero during a reversible process.
C)The entropy change in the surroundings is equal and opposite to that of the system during a reversible process.
D)Reversible change would require an infinite amount of time to complete.
E)Heat transfer between the system and surroundings during phase changes can be approximated as reversible.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
45
The entropy change of the surroundings, Ssurr, is related to heat transfer q with respect to the system and temperature T by
A)(-q/Tsys = Ssurr .)
B)(+q/Tsys = Ssurr .)
C)(-q/Tsurr = Ssurr .)
D)(q/Tsurr = Ssurr .)
E)none of these, unless the system undergoes a reversible process
A)(-q/Tsys = Ssurr .)
B)(+q/Tsys = Ssurr .)
C)(-q/Tsurr = Ssurr .)
D)(q/Tsurr = Ssurr .)
E)none of these, unless the system undergoes a reversible process

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
46
The enthalpy of vaporization of water at 100.0 C is 40.68 kJ/mol.What is the entropy change in the surroundings when one mole of water vapor condenses at 100.0 C in a large room maintained at a temperature of 25.00 C?
A)(-136.4 J/K)
B)(-109.0 J/K)
C)(-40.68 J/K)
D)(+109.0 J/K)
E)(+136.4 J/K)
A)(-136.4 J/K)
B)(-109.0 J/K)
C)(-40.68 J/K)
D)(+109.0 J/K)
E)(+136.4 J/K)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
47
Determine S for the reaction N2O4 (g) ⇄ 2 NO2 (g) given the following information. 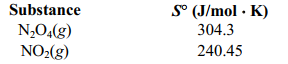
A)(+176.6 J/mol . K)
B)(-63.8 J/mol . K)
C)(+63.8 J/mol . K)
D)(-50.7 J/mol . K)
E)(-176.7 J/mol . K)
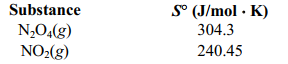
A)(+176.6 J/mol . K)
B)(-63.8 J/mol . K)
C)(+63.8 J/mol . K)
D)(-50.7 J/mol . K)
E)(-176.7 J/mol . K)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
48
What is the entropy change in the surroundings when one mole of ice melts at 0.00 C in a large room maintained at 32.0 C? The heat of fusion of ice is 6.01 kJ/mol.
A)(-188 J/K)
B)(-22.0 J/K)
C)(-19.7 J/K)
D)(+19.7 J/K)
E)(+188 J/K)
A)(-188 J/K)
B)(-22.0 J/K)
C)(-19.7 J/K)
D)(+19.7 J/K)
E)(+188 J/K)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
49
The gas above the liquid in a sealed bottle of soda is primarily carbon dioxide.Carbon dioxide is also dissolved in the soda.When the distribution of carbon dioxide between the gas and liquid is at equilibrium, molecules of carbon dioxide in the gas phase can still dissolve in the liquid phase if they strike the surface and are captured.Similarly, molecules of carbon dioxide can escape from the liquid phase.What is the entropy change of the universe, Suniv, for the dissolution of carbon dioxide under these conditions?
A)( Suniv is less than 0 because the dissolved carbon dioxide has fewer accessible states.)
B)( Suniv is greater than 0 because the dissolved carbon dioxide has fewer accessible states.)
C)( Suniv equals 0 because this is an equilibrium situation.)
D)( Suniv is less than 0 because the gas dissolves spontaneously.)
E)( Suniv is greater than 0 because the gas dissolves spontaneously.)
A)( Suniv is less than 0 because the dissolved carbon dioxide has fewer accessible states.)
B)( Suniv is greater than 0 because the dissolved carbon dioxide has fewer accessible states.)
C)( Suniv equals 0 because this is an equilibrium situation.)
D)( Suniv is less than 0 because the gas dissolves spontaneously.)
E)( Suniv is greater than 0 because the gas dissolves spontaneously.)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
50
Indicate which of the following reactions results in a positive Ssys.
A)Ba(NO3 )2 (aq) + Na2 SO4 (aq) ⇆ BaSO4 (s) + 2 NaNO3 (aq)
B)4 Fe(s) +3 O2 (g) ⇆ 2 Fe2 O3 (s)
C)2 H2 (g) + O2 (g) ⇆ 2 H2 O(g)
D)Zn(s) + 2 HCl(aq) ⇆ ZnCL2 (aq) + H2 (g)
E)2 Na(s) + CL2 (g) ⇆ NaCl(s)
A)Ba(NO3 )2 (aq) + Na2 SO4 (aq) ⇆ BaSO4 (s) + 2 NaNO3 (aq)
B)4 Fe(s) +3 O2 (g) ⇆ 2 Fe2 O3 (s)
C)2 H2 (g) + O2 (g) ⇆ 2 H2 O(g)
D)Zn(s) + 2 HCl(aq) ⇆ ZnCL2 (aq) + H2 (g)
E)2 Na(s) + CL2 (g) ⇆ NaCl(s)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
51
Determine S for the reaction 2 Fe2O3(s) ⇆ 4 Fe (s) + 3O2 (g) given the following information. 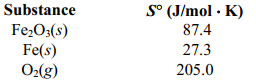
A)(-144.9 J/mol . K)
B)(-549.4 J/mol . K)
C)(+899.0 J/mol . K)
D)(+549.4 J/mol . K)
E)(+144.9 J/mol . K)
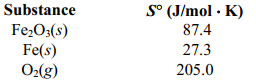
A)(-144.9 J/mol . K)
B)(-549.4 J/mol . K)
C)(+899.0 J/mol . K)
D)(+549.4 J/mol . K)
E)(+144.9 J/mol . K)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
52
When solid pellets of sodium hydroxide (NaOH) dissolve in water, the temperature of the water can rise dramatically.Taking NaOH as the system, what can you deduce about the signs of the entropy change of the system ( Ssys) and surroundings ( Ssurr) from this?
A)( Ssys is less than 0 and Ssurr is less than 0.)
B)( Ssys is less than 0 and Ssurr is greater than 0.)
C)( Ssys is greater than 0 and Ssurr is less than 0.)
D)( Ssys is greater than 0 and Ssurr is greater than 0.)
E)Nothing can be deduced from this limited information.
A)( Ssys is less than 0 and Ssurr is less than 0.)
B)( Ssys is less than 0 and Ssurr is greater than 0.)
C)( Ssys is greater than 0 and Ssurr is less than 0.)
D)( Ssys is greater than 0 and Ssurr is greater than 0.)
E)Nothing can be deduced from this limited information.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
53
When solid ammonium nitrate (NH4NO3) dissolves in water, the temperature of the water can drop dramatically.Taking NH4NO3 as the system, predict the signs of the entropy change of the system ( Ssys) and surroundings ( Ssurr).
A)( Ssys is less than 0 and Ssurr is less than 0.)
B)( Ssys is less than 0 and Ssurr is greater than 0.)
C)( Ssys is greater than 0 and Ssurr is less than 0.)
D)( Ssys is greater than 0 and Ssurr is greater than 0.)
E)( Ssys equals 0 and Ssurr is less than 0.)
A)( Ssys is less than 0 and Ssurr is less than 0.)
B)( Ssys is less than 0 and Ssurr is greater than 0.)
C)( Ssys is greater than 0 and Ssurr is less than 0.)
D)( Ssys is greater than 0 and Ssurr is greater than 0.)
E)( Ssys equals 0 and Ssurr is less than 0.)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
54
The enthalpy and entropy of vaporization of water at 100.00 C are 40.66 kJ/mol and 109.0 J/mol . K, respectively.What is the entropy change of the universe when one mole of steam condenses in a large room maintained at 25.00 C? Assume the final temperature of the water is 100.00 C.
A)(+136.4 J/K)
B)(-136.4 J/K)
C)(+27.4 J/K)
D)(-27.4 J/K)
E)(+245.4 J/K)
A)(+136.4 J/K)
B)(-136.4 J/K)
C)(+27.4 J/K)
D)(-27.4 J/K)
E)(+245.4 J/K)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
55
The enthalpy and entropy of vaporization of acetone at its normal boiling point, -56.4 C, are 31.3 kJ/mol and 95.0 J/mol . K, respectively.What would the entropy change of the universe be if one mole of acetone vapor were to condense in a large room maintained at 25.0 C?
A)(-2.0 * 102 J/K)
B)(+10.0 J/K)
C)(+1.05 * 102 J/K)
D)(-10.0 J/K)
E)(+2.0 * 102 J/K)
A)(-2.0 * 102 J/K)
B)(+10.0 J/K)
C)(+1.05 * 102 J/K)
D)(-10.0 J/K)
E)(+2.0 * 102 J/K)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following processes are reversible in the thermodynamic sense? I.Iron in the open air rusts.
II.NaCl is dissolved in water and then recovered by the evaporation of the water.
III.The ice in a mixture of ice and water at 0 C and 1 atm melts.
A)I only
B)II only
C)III only
D)II and III only
E)I, II, and III are all reversible.
II.NaCl is dissolved in water and then recovered by the evaporation of the water.
III.The ice in a mixture of ice and water at 0 C and 1 atm melts.
A)I only
B)II only
C)III only
D)II and III only
E)I, II, and III are all reversible.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
57
The enthalpy and entropy of vaporization of ammonia at its normal boiling point, -33.34 C, are 23.35 kJ/mol and 97.4 J/mol . K, respectively.What is the entropy change of the universe when one mole of liquid ammonia vaporizes in a large room maintained at 34.0 C? Assume the final temperature of the ammonia gas is -33.34 C.
A)(+21.4 J/K)
B)(-76.0 J/K)
C)(+74.1 J/K)
D)(-21.4 J/K)
E)(+76.0 J/K)
A)(+21.4 J/K)
B)(-76.0 J/K)
C)(+74.1 J/K)
D)(-21.4 J/K)
E)(+76.0 J/K)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
58
Ssys can be directly related to the heat q.Which of the following statements is NOT true?
A)( Ssys can always be determined from the heat transferred during the actual process.)
B)For a reversible spontaneous endothermic process, both q and Ssys will be positive.
C)The greater the amount of heat transferred, the larger the magnitude of the entropy change.
D)The higher the temperature at which heat is transferred, the lower the entropy change.
E)All of these statements are true.
A)( Ssys can always be determined from the heat transferred during the actual process.)
B)For a reversible spontaneous endothermic process, both q and Ssys will be positive.
C)The greater the amount of heat transferred, the larger the magnitude of the entropy change.
D)The higher the temperature at which heat is transferred, the lower the entropy change.
E)All of these statements are true.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
59
Determine S for the reaction Zn(s) + 2 HCl (aq) ⇄ ZnCL2 (aq) +H2 (g) given the following information: 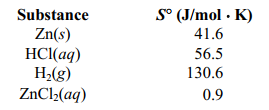
A)0 J/K
B)(-23.1 J/K)
C)(+23.1 J/K)
D)(-33.4 J/K)
E)(+33.4 J/K)
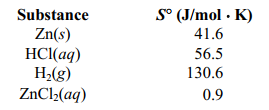
A)0 J/K
B)(-23.1 J/K)
C)(+23.1 J/K)
D)(-33.4 J/K)
E)(+33.4 J/K)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
60
Heat transfer from the system to the surroundings has a large effect on Ssurr
A)when the temperature of the surroundings is low.
B)when the temperature of the surroundings is high.
C)when the temperature of the system is low.
D)when the temperature of the system is high.
E)at any temperature, as the amount of heat transferred is independent of temperature.
A)when the temperature of the surroundings is low.
B)when the temperature of the surroundings is high.
C)when the temperature of the system is low.
D)when the temperature of the system is high.
E)at any temperature, as the amount of heat transferred is independent of temperature.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
61
Determine the value of G at 25 C for the reaction O3(g) +N2 O(g) ⇄ O2(g) + 2 NO(g) given the following information:
A)(-94.2 kJ)
B)(+5.8 kJ)
C)(+50.0 kJ)
D)(-50.0 kJ)
E)(-40.0 kJ)

A)(-94.2 kJ)
B)(+5.8 kJ)
C)(+50.0 kJ)
D)(-50.0 kJ)
E)(-40.0 kJ)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
62
Zinc sulfide can be oxidized by oxygen gas according to the reaction 2 ZnS(s) +O2 (g) ? 2 ZnO(s) + S(s).If H =-289.0 kJ/mol and S =-169.4 J/K.mol, what is G For this reaction at 25.00 C?
A)(-284.8 kJ/mol)
B)(-238.5 kJ/mol)
C)339.5 kJ/mol
D)50.80 kJ/mol
E)(-339.5 kJ/mol)
A)(-284.8 kJ/mol)
B)(-238.5 kJ/mol)
C)339.5 kJ/mol
D)50.80 kJ/mol
E)(-339.5 kJ/mol)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
63
Methane and water vapor can react to form carbon monoxide and hydrogen gas according to the reaction CH4 (g) + H2O(g) ⇄ CO(g) +3 H2 (g).If H = 206.1 kJ/mol and S = 214.5 J/mol .K, what is G for this reaction at 25.00 C?
A)(-6.375 *104 kJ/mol)
B)(-8.400 kJ/mol)
C)142.1 kJ/mol
D)205.9 kJ/mol
E)(-5156 kJ/mol)
A)(-6.375 *104 kJ/mol)
B)(-8.400 kJ/mol)
C)142.1 kJ/mol
D)205.9 kJ/mol
E)(-5156 kJ/mol)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
64
At constant T and P, any reaction will be spontaneous if
A)( Gsys 0.)
B)( Gsys 0.)
C)( Ssys 0.)
D)( Ssys 0.)
E)( Hsys 0.)
A)( Gsys 0.)
B)( Gsys 0.)
C)( Ssys 0.)
D)( Ssys 0.)
E)( Hsys 0.)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
65
Estimate the standard molar entropy of 1.00 M aqueous Mg2+ using the following information: S for the reaction Mg(s) + 2 HCl(aq) ⇄ MgCL2 (aq) +H2 (g) is -43.02 J/K. 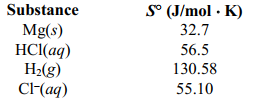
A)(-52.1 J/K)
B)(+140.J/K)
C)(+138 J/K)
D)(-138 J/K)
E)(-140.J/K)
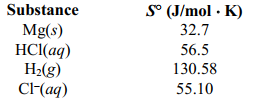
A)(-52.1 J/K)
B)(+140.J/K)
C)(+138 J/K)
D)(-138 J/K)
E)(-140.J/K)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
66
The symbol G f NO2, (g) refers to which of the following reactions?
A)NO2(l) NO2(g)
B)NO(g) +O(g) NO2(g)
C)N(g) + O2(g) NO2(g)
D)N(g)+2 O(g) NO2(g)
E)1/2 N2(g) +O2(g) NO2(g)
A)NO2(l) NO2(g)
B)NO(g) +O(g) NO2(g)
C)N(g) + O2(g) NO2(g)
D)N(g)+2 O(g) NO2(g)
E)1/2 N2(g) +O2(g) NO2(g)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
67
Processes are always spontaneous when _______ (H and S refer to the system).
A)( H > 0 and S < 0
B)( H 0 and S < 0
C)( H > 0 and S 0
D)( H 0 and S 0
E)(None of these is true, as temperature must always be taken into account.
A)( H > 0 and S < 0
B)( H 0 and S < 0
C)( H > 0 and S 0
D)( H 0 and S 0
E)(None of these is true, as temperature must always be taken into account.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
68
For the dimerization of nitrogen dioxide to form dinitrogen tetroxide (N2O4) according to the reaction 2 NO2 (g) ⇄ N2 O4 (g), the value of H is -57.2 kJ/mol, and that of S is -175.8 J/mol . K.Determine G at 25.00 C.
A)(+5.236 * 104 kJ/mol)
B)(-52.8 kJ/mol)
C)(-4.8 kJ/mol)
D)(-109.6 kJ/mol)
E)(-61.6 kJ/mol)
A)(+5.236 * 104 kJ/mol)
B)(-52.8 kJ/mol)
C)(-4.8 kJ/mol)
D)(-109.6 kJ/mol)
E)(-61.6 kJ/mol)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
69
Determine Grxn for C4 H10 (l) +13/2 O2 (g) 4 CO2(g) + 5 H2O(g) given the following information.
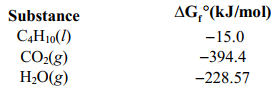
A)(-2705.5 kJ/mol)
B)(-608.0 kJ/mol)
C)(-1791.0 kJ/mol)
D)(-2735.5 kJ/mol)
E)(+608.0 kJ/mol)
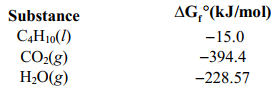
A)(-2705.5 kJ/mol)
B)(-608.0 kJ/mol)
C)(-1791.0 kJ/mol)
D)(-2735.5 kJ/mol)
E)(+608.0 kJ/mol)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
70
For the dimerization of nitrogen dioxide to form dinitrogen tetroxide (N2O4) according to the reaction 2 NO2 (g) ⇄ N2 O4 (g), the value of H is -57.2 kJ/mol, and that of S is -175.8 J/mol . K.Estimate
G at 100.00 C.
A)(+6.6 kJ/mol)
B)(-39.6 kJ/mol)
C)(-122.8 kJ/mol)
D)(-74.8 kJ/mol)
E)(+8.4 kJ/mol)
G at 100.00 C.
A)(+6.6 kJ/mol)
B)(-39.6 kJ/mol)
C)(-122.8 kJ/mol)
D)(-74.8 kJ/mol)
E)(+8.4 kJ/mol)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
71
Determine the standard entropy change of the universe at 25 C for the reaction
NH4 + (aq) + Cl - (aq) NH4 Cl(s) given the following information.Is the reaction spontaneous under Standard conditions?
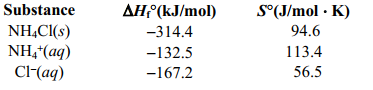
A)(-26.0 J/K, no)
B)(+49.3 J/K, yes)
C)(-75.3 J/K, no)
D)(+124.6 J/K, yes)
E)(+26.0 J/K, yes)
NH4 + (aq) + Cl - (aq) NH4 Cl(s) given the following information.Is the reaction spontaneous under Standard conditions?
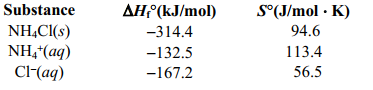
A)(-26.0 J/K, no)
B)(+49.3 J/K, yes)
C)(-75.3 J/K, no)
D)(+124.6 J/K, yes)
E)(+26.0 J/K, yes)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
72
At body temperature, many proteins have a well-defined structure that is essential to their function.As the temperature is raised, however, the structure changes and the protein no longer functions.What can be deduced from this information about the signs of the enthalpy and entropy changes for denaturation?
A)( H 0 and S 0)
B)( H 0 and S 0)
C)( H 0 and S 0)
D)( H 0 and S 0)
E)There is insufficient information to deduce anything about the signs of H and S
A)( H 0 and S 0)
B)( H 0 and S 0)
C)( H 0 and S 0)
D)( H 0 and S 0)
E)There is insufficient information to deduce anything about the signs of H and S

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
73
Calculate the maximum amount of work that can be done by the reaction CH4(g) + 2 O2(g) ⇄ CO2(g)+2 H2O(g) given the following information.
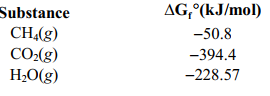
A)(-673.8 kJ/mol)
B)(-750.9 kJ/mol)
C)(-902.3 kJ/mol)
D)(-216.6 kJ/mol)
E)(-800.7 kJ/mol)
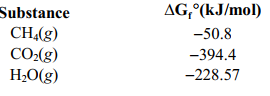
A)(-673.8 kJ/mol)
B)(-750.9 kJ/mol)
C)(-902.3 kJ/mol)
D)(-216.6 kJ/mol)
E)(-800.7 kJ/mol)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
74
The dissolution of ammonium nitrate in water is a spontaneous endothermic process.It is spontaneous because the system undergoes
A)a decrease in enthalpy.
B)an increase in entropy.
C)an increase in enthalpy.
D)a decrease in entropy.
E)an increase in free energy.
A)a decrease in enthalpy.
B)an increase in entropy.
C)an increase in enthalpy.
D)a decrease in entropy.
E)an increase in free energy.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following statements regarding free energy, work, and efficiency is NOT correct?
A)The thermal energy released during combustion reactions can be completely converted into useful work.
B)The enthalpy change associated with a chemical reaction can theoretically be divided into the energy available to do useful work and the energy that spreads out.
C)In the free energy equation, the T S term equals the P-V work done by the system.
D)For a nonspontaneous reaction to occur, work must be done on the reaction system.
E)Maximum efficiency requires that a reaction occur infinitely slowly and reversibly.
A)The thermal energy released during combustion reactions can be completely converted into useful work.
B)The enthalpy change associated with a chemical reaction can theoretically be divided into the energy available to do useful work and the energy that spreads out.
C)In the free energy equation, the T S term equals the P-V work done by the system.
D)For a nonspontaneous reaction to occur, work must be done on the reaction system.
E)Maximum efficiency requires that a reaction occur infinitely slowly and reversibly.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the relationships between the free energy change of a system and associated entropy changes is true?
A)( Gsys = +T Ssys)
B)( Gsys =-T Ssys)
C)( Gsys =+T Suniv)
D)( Gsys = -T Ssurr)
E)( Gsys = -T Suniv)
A)( Gsys = +T Ssys)
B)( Gsys =-T Ssys)
C)( Gsys =+T Suniv)
D)( Gsys = -T Ssurr)
E)( Gsys = -T Suniv)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
77
Consider substances that exist as liquids under standard state conditions.What must be the relationship between the enthalpy and free energy of formation for the liquid and the gaseous form of the substance? (Read as more negative and as less negative.)
A)Hf0(l) Hf0(g) and Gf0(l) Gf0 (g)
B)Hf0(l) Hf0(g) and Gf0(l) Gf0 (g)
C)Hf0(l) Hf0(g) and Gf0(l) Gf0 (g)
D)Hf0(l) Hf0(g) and Gf0(l) Gf0 (g)
E)No strict relationship between these values applies.
A)Hf0(l) Hf0(g) and Gf0(l) Gf0 (g)
B)Hf0(l) Hf0(g) and Gf0(l) Gf0 (g)
C)Hf0(l) Hf0(g) and Gf0(l) Gf0 (g)
D)Hf0(l) Hf0(g) and Gf0(l) Gf0 (g)
E)No strict relationship between these values applies.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
78
Indicate which of the following reactions results in a negative Ssys.
A)AgNO3 (aq) + NaCl(aq) ⇄ AgCl(s) + NaCl(aq)
B)2 C2 H6 (g) +7 O2 (g) ⇄ 4 CO2 (g) +6 H2 O(g)
C)MgSO4 . 7 H2 O(s) ⇄ MgSO4 (s) +7 H2 O(g)
D)N2 O4 (g) ⇄ 2 NO2 (g)
E)Fe(OH)3 (s) + 3H+(aq) ⇄ 3H2O(l) + Fe3+(aq)
A)AgNO3 (aq) + NaCl(aq) ⇄ AgCl(s) + NaCl(aq)
B)2 C2 H6 (g) +7 O2 (g) ⇄ 4 CO2 (g) +6 H2 O(g)
C)MgSO4 . 7 H2 O(s) ⇄ MgSO4 (s) +7 H2 O(g)
D)N2 O4 (g) ⇄ 2 NO2 (g)
E)Fe(OH)3 (s) + 3H+(aq) ⇄ 3H2O(l) + Fe3+(aq)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
79
Hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH) to form sodium chloride (NaCl) and water.If H =-56.13 kJ/mol and S =79.11 J/mol. K, what is G for this reaction at 20.00 C?
A)(-79.32 kJ/mol)
B)(-77.73 kJ/mol)
C)(-2.324 *104 kJ/mol)
D)79.32 kJ/mol
E)(-1638 kJ/mol)
A)(-79.32 kJ/mol)
B)(-77.73 kJ/mol)
C)(-2.324 *104 kJ/mol)
D)79.32 kJ/mol
E)(-1638 kJ/mol)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following statements regarding free energy is NOT true?
A)Free energy represents the maximum work that a thermodynamic system can perform during a spontaneous change.
B)Free energy represents the minimum work that would be required to make a thermodynamic system undergo a nonspontaneous change.
C)The free energy of a system increases during the course of a spontaneous reaction as the entropy of the universe increases.
D)The Gibbs free energy change of a reaction reflects the work associated with processes happening at constant temperature and pressure.
E)The Gibbs free energy change of a spontaneous process is negative.
A)Free energy represents the maximum work that a thermodynamic system can perform during a spontaneous change.
B)Free energy represents the minimum work that would be required to make a thermodynamic system undergo a nonspontaneous change.
C)The free energy of a system increases during the course of a spontaneous reaction as the entropy of the universe increases.
D)The Gibbs free energy change of a reaction reflects the work associated with processes happening at constant temperature and pressure.
E)The Gibbs free energy change of a spontaneous process is negative.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck



