Deck 19: Organic Chemistry Fuels, Pharmaceuticals, and Modern Materials
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/145
Play
Full screen (f)
Deck 19: Organic Chemistry Fuels, Pharmaceuticals, and Modern Materials
1
Organic chemistry encompasses the chemistry of all
A)hydrocarbons.
B)functional groups.
C)monomers, oligomers, and polymers.
D)naturally occurring compounds.
E)carbon-containing compounds.
A)hydrocarbons.
B)functional groups.
C)monomers, oligomers, and polymers.
D)naturally occurring compounds.
E)carbon-containing compounds.
carbon-containing compounds.
2
Without working out all the structures, which has the larger number of structural isomers, C14H30 or C15H32?
A)C14H30
B)C15H32
C)They have the same number of isomers.
D)There is no way to tell without working out all the structures.
E)It depends on the number of multiple bonds in the molecule.
A)C14H30
B)C15H32
C)They have the same number of isomers.
D)There is no way to tell without working out all the structures.
E)It depends on the number of multiple bonds in the molecule.
C15H32
3
A hydrocarbon is a compound that contains
A)carbon with hydrogen and oxygen in the ratio 2:1.
B)carbon with hydrogen and oxygen in any ratio.
C)only carbon and hydrogen.
D)carbon, hydrogen, and any functional groups.
E)only carbon, hydrogen, and oxygen.
A)carbon with hydrogen and oxygen in the ratio 2:1.
B)carbon with hydrogen and oxygen in any ratio.
C)only carbon and hydrogen.
D)carbon, hydrogen, and any functional groups.
E)only carbon, hydrogen, and oxygen.
only carbon and hydrogen.
4
Which of the following statements regarding hydrocarbons is NOT correct?
A)Alkanes contain carbons that are sp3 hybridized.
B)Alkynes contain at least one carbon-carbon double bond.
C)The carbons in the functional group characterizing an alkene are sp2 hybridized.
D)The general chemical formula for alkanes is CnH2n+2.
E)The general formula for cycloalkanes is CnH2n.
A)Alkanes contain carbons that are sp3 hybridized.
B)Alkynes contain at least one carbon-carbon double bond.
C)The carbons in the functional group characterizing an alkene are sp2 hybridized.
D)The general chemical formula for alkanes is CnH2n+2.
E)The general formula for cycloalkanes is CnH2n.

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
5
The C - C - C bond angles in octane CH3CH2CH2CH2CH2CH2CH2CH3) are all
A)180 .
B)120 .
C)90 .
D)109.5 .
E)60 .
A)180 .
B)120 .
C)90 .
D)109.5 .
E)60 .

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
6
The basic building block of a polymer is called
A)a functional group.
B)a monomer.
C)a monotone.
D)an oligomer.
E)an addition.
A)a functional group.
B)a monomer.
C)a monotone.
D)an oligomer.
E)an addition.

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
7
What is NOT a correct formula for the compound illustrated below? 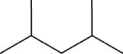
A)CH3CH2)5CH3
B)CH3)2CHCH2CHCH3)2
C)CH3CHCH3)CH2CHCH3)CH3
D)C7H16
E)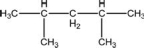

A)CH3CH2)5CH3
B)CH3)2CHCH2CHCH3)2
C)CH3CHCH3)CH2CHCH3)CH3
D)C7H16
E)


Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following are true regarding cyclohexane, whose carbon skeleton is shown? 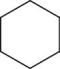
I.The molecule is flat.
II.Each carbon is bonded to four other atoms.
III.Each carbon is sp3 hybridized.
A)I only
B)II only
C)III only
D)II and III only
E)I, II, and III are all true.

I.The molecule is flat.
II.Each carbon is bonded to four other atoms.
III.Each carbon is sp3 hybridized.
A)I only
B)II only
C)III only
D)II and III only
E)I, II, and III are all true.

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following is a methylene group?
A)(-CH2)
B)(=CH2)
C)(-CH2-)
D)(=CH=)
E)(-CH3)
A)(-CH2)
B)(=CH2)
C)(-CH2-)
D)(=CH=)
E)(-CH3)

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
10
Which is NOT true about n-alkanes?
A)Their formulas are all CnH2n2.
B)All the carbon atoms are centers of tetrahedral geometry.
C)Their melting points increase with the number of carbon atoms.
D)Their boiling points decrease with the number of carbon atoms.
E)All of the carbons are sp3 hybridized.
A)Their formulas are all CnH2n2.
B)All the carbon atoms are centers of tetrahedral geometry.
C)Their melting points increase with the number of carbon atoms.
D)Their boiling points decrease with the number of carbon atoms.
E)All of the carbons are sp3 hybridized.

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following tends to be true of polymers but not small molecules?
I.variable rather than constant composition
II.melts over a range of temperatures poorly defined melting point)
III.solid state regions that are disordered
A)I
B)II
C)III
D)I, II, and III
E)None of these, as polymers are molecules just like small molecules, only bigger.
I.variable rather than constant composition
II.melts over a range of temperatures poorly defined melting point)
III.solid state regions that are disordered
A)I
B)II
C)III
D)I, II, and III
E)None of these, as polymers are molecules just like small molecules, only bigger.

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
12
A primary difference between branched chain alkanes and normal alkanes is
A)the ratio of hydrogen to carbon.
B)the number of hydrogen atoms bonded to nonterminal carbon atoms.
C)the orbital hybridization of the carbon atoms.
D)the bond angles around each carbon atom.
E)the degree to which electrons are delocalized.
A)the ratio of hydrogen to carbon.
B)the number of hydrogen atoms bonded to nonterminal carbon atoms.
C)the orbital hybridization of the carbon atoms.
D)the bond angles around each carbon atom.
E)the degree to which electrons are delocalized.

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
13
Iso-octane has the following structure.In this structure, how many carbon atoms are bonded to only two other carbon atoms? 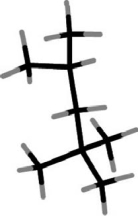
A)0
B)1
C)2
D)3
E)4

A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
14
The -CH3 unit is known as
A)a methyl group.
B)a methylene group.
C)an acetylene group.
D)an alkyne group.
E)an alkene group.
A)a methyl group.
B)a methylene group.
C)an acetylene group.
D)an alkyne group.
E)an alkene group.

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
15
Which pair shows two different molecules?
A)CH3CH2)2CCH3)3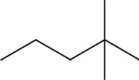
B)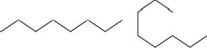
C)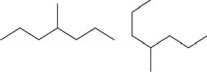
D)CH3)2CHCHCH3)CHCH3)2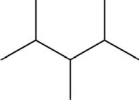
E)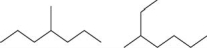
A)CH3CH2)2CCH3)3

B)

C)

D)CH3)2CHCHCH3)CHCH3)2

E)


Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
16
A subunit of an organic compound that confers particular chemical and physical properties to that compound is termed
A)a monomer.
B)an oligomer.
C)a functional group.
D)a synthetic unit.
E)an isomer.
A)a monomer.
B)an oligomer.
C)a functional group.
D)a synthetic unit.
E)an isomer.

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
17
Determine the number of carbons in the longest chain for the following molecule. 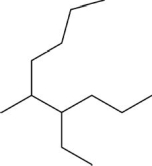
A)6
B)7
C)8
D)9
E)10

A)6
B)7
C)8
D)9
E)10

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
18
What is the molecular formula for the compound illustrated below? 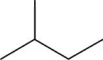
A)C5H8
B)C5H9
C)C5H10
D)C5H11
E)C5H12

A)C5H8
B)C5H9
C)C5H10
D)C5H11
E)C5H12

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following molecules would NOT be considered "organic"?
A)CaC2
B)CH3NH2
C)(CH3)2O
D)NaCH3(CH2)4COO
E)(NH2)2CO
A)CaC2
B)CH3NH2
C)(CH3)2O
D)NaCH3(CH2)4COO
E)(NH2)2CO

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following is true about normal alkanes?
A)They all exhibit strong hydrogen bonding with one another and with water.
B)Some are gases, some are liquids, and some are solids at room temperature.
C)They all contain carbon, hydrogen, and oxygen in consistent ratios.
D)They all contain at least one double bond.
E)They all have the formula CnH2n.
A)They all exhibit strong hydrogen bonding with one another and with water.
B)Some are gases, some are liquids, and some are solids at room temperature.
C)They all contain carbon, hydrogen, and oxygen in consistent ratios.
D)They all contain at least one double bond.
E)They all have the formula CnH2n.

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
21
In a hydrogenation experiment, 50.00 grams of a straight-chain hydrocarbon containing 8 carbon atoms reacts with 1.829 grams of hydrogen gas.The hydrocarbon probably has the chemical formula
A)C8H18.
B)C8H16.
C)C8H14.
D)C8H10.
E)Insufficient information is provided.
A)C8H18.
B)C8H16.
C)C8H14.
D)C8H10.
E)Insufficient information is provided.

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
22
Name the compound with the structural formula CH3(CH2)16CH3.
A)n-octane
B)n-hexadecane
C)n-octadecane
D)n-decane
E)1,16-dimethylhexadecane
A)n-octane
B)n-hexadecane
C)n-octadecane
D)n-decane
E)1,16-dimethylhexadecane

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
23
What is the degree of unsaturation of the following molecule? 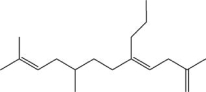
A)1
B)2
C)3
D)4
E)5

A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
24
Crude oil is composed mostly of
A)liquefied carbon.
B)liquefied rock and soil.
C)compounds composed of carbon and liquid alkanes.
D)decomposed anaerobic bacteria.
E)compounds composed of carbon, hydrogen, oxygen, and nitrogen.
A)liquefied carbon.
B)liquefied rock and soil.
C)compounds composed of carbon and liquid alkanes.
D)decomposed anaerobic bacteria.
E)compounds composed of carbon, hydrogen, oxygen, and nitrogen.

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
25
Cyclic alkanes differ from normal alkanes in
A)the ratio of hydrogen to carbon.
B)their utility as a combustible fuel.
C)the orbital hybridization on the carbon atoms.
D)the number of hydrogens bonded to nonterminal carbon atoms.
E)the number of double bonds.
A)the ratio of hydrogen to carbon.
B)their utility as a combustible fuel.
C)the orbital hybridization on the carbon atoms.
D)the number of hydrogens bonded to nonterminal carbon atoms.
E)the number of double bonds.

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
26
What is the molecular formula for the following compound? 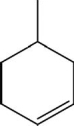
A)C6H11
B)C6H12
C)C7H11
D)C7H12
E)C7H14

A)C6H11
B)C6H12
C)C7H11
D)C7H12
E)C7H14

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
27
Most simple ionic compounds that are soluble in water are not soluble in organic solvents.However, replacing a common cation such as Na+ with an alkyl ammonium ion with four alkyl groups greatly enhances solubility of the ionic compound in many organic solvents.Which formula represents the tetrabutylammonium ion that is found in tetrabutylammonium bromide?
A)[CH3)4N]+
B)[CH3CH2)4N]+
C)[CH3CH2)2)4N]+
D)[CH3CH2)3)4N]+
E)[CCH3)3NH2]+
A)[CH3)4N]+
B)[CH3CH2)4N]+
C)[CH3CH2)2)4N]+
D)[CH3CH2)3)4N]+
E)[CCH3)3NH2]+

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
28
What is the formula for n-nonane?
A)CH3(CH2)9CH3
B)CH3(CH2)17CH3
C)CH3(CH2)7CH3
D)CH3(CH2)5CH3
E)CH3(CH2)8CH3
A)CH3(CH2)9CH3
B)CH3(CH2)17CH3
C)CH3(CH2)7CH3
D)CH3(CH2)5CH3
E)CH3(CH2)8CH3

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
29
When 1 mol of an alkyne with two degrees of unsaturation is hydrogenated, how many moles of hydrogen gas react?
A)0 mol
B)1.5 mol
C)0.5 mol
D)1.0 mol
E)2.0 mol
A)0 mol
B)1.5 mol
C)0.5 mol
D)1.0 mol
E)2.0 mol

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
30
What is the degree of unsaturation of the following molecule? 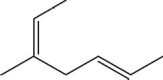
A)1
B)2
C)3
D)4
E)5

A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
31
Of an unknown compound containing only carbon and hydrogen, 0.123 moles was found to stoichiometrically react with 0.246 moles of hydrogen gas.Based on these data, we know that the unknown compound must be
A)an alkane.
B)an alkene.
C)an alkyne.
D)either an alkane or an alkene.
E)either an alkene or an alkyne.
A)an alkane.
B)an alkene.
C)an alkyne.
D)either an alkane or an alkene.
E)either an alkene or an alkyne.

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following is NOT likely to be a component of a natural gas deposit?
A)CH4
B)C3H8
C)C4H8
D)C4H10
E)C8H18
A)CH4
B)C3H8
C)C4H8
D)C4H10
E)C8H18

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
33
A compound composed solely of carbon and hydrogen that is unsaturated must be______ .Choose the best answer.
A)an alkane
B)an alkene
C)an alkyne
D)either an alkane or an alkene
E)either an alkene or an alkyne
A)an alkane
B)an alkene
C)an alkyne
D)either an alkane or an alkene
E)either an alkene or an alkyne

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
34
A saturated compound composed solely of carbon and hydrogen must be
A)an alkane.
B)an alkene.
C)an alkyne.
D)either an alkane or an alkene.
E)either an alkene or an alkyne.
A)an alkane.
B)an alkene.
C)an alkyne.
D)either an alkane or an alkene.
E)either an alkene or an alkyne.

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
35
What is the systematic name of the molecule shown? 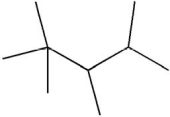
A)2,2,3,4-tetramethylpentane
B)2,3,4,4-tetramethylpentane
C)1,2,3,4,4-pentamethylbutane
D)1,1,1,2,3,3-hexamethylpropane
E)2-tert-butyl-3-methylbutane

A)2,2,3,4-tetramethylpentane
B)2,3,4,4-tetramethylpentane
C)1,2,3,4,4-pentamethylbutane
D)1,1,1,2,3,3-hexamethylpropane
E)2-tert-butyl-3-methylbutane

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following statements regarding alkenes and alkynes is NOT correct?
A)Alkenes and alkynes are typically present in large amounts in crude oil.
B)Alkenes are commonly derived from plant sources.
C)Alkynes are not common in nature due to the reactivity of the CC bond.
D)Alkenes and alkynes can be hydrogenated to give alkanes.
E)Hydrocarbon reactivity increases in the order alkanes alkenes alkynes.
A)Alkenes and alkynes are typically present in large amounts in crude oil.
B)Alkenes are commonly derived from plant sources.
C)Alkynes are not common in nature due to the reactivity of the CC bond.
D)Alkenes and alkynes can be hydrogenated to give alkanes.
E)Hydrocarbon reactivity increases in the order alkanes alkenes alkynes.

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
37
What is the systematic name of the molecule shown? 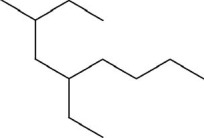
A)3-methyl-5-butylheptane
B)2,4-diethyloctane
C)2-methyl-4-ethylhexane
D)3-methyl-5-ethylnonane
E)4-ethyl-7-methylnonane

A)3-methyl-5-butylheptane
B)2,4-diethyloctane
C)2-methyl-4-ethylhexane
D)3-methyl-5-ethylnonane
E)4-ethyl-7-methylnonane

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
38
What is the systematic name of the molecule shown? 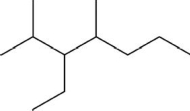
A)4-methyl-5-isopropylheptane
B)3-propyl-4-methylheptane
C)2-methyl-3,4-diethylpentane
D)2,4-dimethyl-3-ethylheptane
E)3-ethyl-2,4-dimethylheptane

A)4-methyl-5-isopropylheptane
B)3-propyl-4-methylheptane
C)2-methyl-3,4-diethylpentane
D)2,4-dimethyl-3-ethylheptane
E)3-ethyl-2,4-dimethylheptane

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following statements regarding the reactions of alkanes, alkenes, and alkynes is NOT correct?
A)The electrons in the bonds of alkenes and alkynes are more likely to react than electrons in the bonds in alkanes.
B)Alkenes and alkynes readily undergo addition reactions with molecules such as HCl, HBr, and HI.
C)An example of an addition reaction involving an alkene is H2C = CH2 +HCl H3C-CH2Cl.
D)Alkenes and alkynes are important intermediates in the syntheses of other organic molecules.
E)Alkanes readily undergo addition reactions with highly reactive molecules such as F2.
A)The electrons in the bonds of alkenes and alkynes are more likely to react than electrons in the bonds in alkanes.
B)Alkenes and alkynes readily undergo addition reactions with molecules such as HCl, HBr, and HI.
C)An example of an addition reaction involving an alkene is H2C = CH2 +HCl H3C-CH2Cl.
D)Alkenes and alkynes are important intermediates in the syntheses of other organic molecules.
E)Alkanes readily undergo addition reactions with highly reactive molecules such as F2.

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
40
Of an unknown compound containing only carbon and hydrogen, 0.123 moles of the compound was found to stoichiometrically react with 0.123 moles of hydrogen gas.Based on these data, we know that the unknown compound must be
A)an alkane.
B)an alkene.
C)an alkyne.
D)either an alkane or an alkene.
E)either an alkene or an alkyne.
A)an alkane.
B)an alkene.
C)an alkyne.
D)either an alkane or an alkene.
E)either an alkene or an alkyne.

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
41
Name the compound. 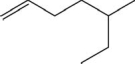
A)3-methyl-6-heptene
B)5-ethyl-1-hexene
C)5-methyl-1-heptene
D)2-ethyl-5-hexene
E)3-methylpentylethylene

A)3-methyl-6-heptene
B)5-ethyl-1-hexene
C)5-methyl-1-heptene
D)2-ethyl-5-hexene
E)3-methylpentylethylene

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
42
Do carbon atoms in aromatic hydrocarbon molecules not ions) obey the octet rule?
A)No, each carbon is bonded to only three atoms, and there are no lone pairs.
B)No, each carbon has one single bond and one double bond for a total of only six electrons.
C)Yes, each carbon obeys the octet rule.
D)Yes, but only because the double bond is counted twice owing to the resonance structures.
E)Yes, but only when one or the other resonance form dominates, not when the structure is an average of the two.
A)No, each carbon is bonded to only three atoms, and there are no lone pairs.
B)No, each carbon has one single bond and one double bond for a total of only six electrons.
C)Yes, each carbon obeys the octet rule.
D)Yes, but only because the double bond is counted twice owing to the resonance structures.
E)Yes, but only when one or the other resonance form dominates, not when the structure is an average of the two.

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
43
Are the methyl groups in xylene, C6H4(CH3)2, included in the aromatic part of the molecular structure?
A)Yes, the aromatic bonding extends throughout the molecule.
B)Yes, but only when the resonance form with double bonds to the methyl groups exists.
C)No, only the carbons in the ring are involved in the aromatic system.
D)No, only the hydrogens in the ring are involved in the aromatic bonding.
E)No, only the carbon atoms in xylene are involved in aromatic bonding, not the hydrogens.
A)Yes, the aromatic bonding extends throughout the molecule.
B)Yes, but only when the resonance form with double bonds to the methyl groups exists.
C)No, only the carbons in the ring are involved in the aromatic system.
D)No, only the hydrogens in the ring are involved in the aromatic bonding.
E)No, only the carbon atoms in xylene are involved in aromatic bonding, not the hydrogens.

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
44
Consider the 1,2-dichloro derivative of ethylene in which two of the hydrogen atoms on one of the carbon atoms in ethylene is substituted with a chlorine atom, giving the structural formula CH2=CCl2.Does this molecule have cis-trans isomers? Remember that ethylene does not.
A)yes, because the chlorines can be placed on the same or opposite sides of the double bond
B)yes, because the chlorines are placed on a single carbon
C)no, because both the formula and bonds are the same in all skeletal structures
D)no, because the two structures can interconvert through bond rotation
E)no, because there are less than four atoms bound to each carbon
A)yes, because the chlorines can be placed on the same or opposite sides of the double bond
B)yes, because the chlorines are placed on a single carbon
C)no, because both the formula and bonds are the same in all skeletal structures
D)no, because the two structures can interconvert through bond rotation
E)no, because there are less than four atoms bound to each carbon

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following statements regarding polymers is NOT correct?
A)Polymers often do not have a sharp melting point.
B)Polymers cannot contain double bonds because they are too reactive.
C)Branched-chain polymers tend to be more flexible than straight-chain polymers.
D)Straight-chain polymers tend to have higher densities than branched-chain polymers.
E)Heteroatoms in polymers can drastically change the properties of a hydrocarbon polymer.
A)Polymers often do not have a sharp melting point.
B)Polymers cannot contain double bonds because they are too reactive.
C)Branched-chain polymers tend to be more flexible than straight-chain polymers.
D)Straight-chain polymers tend to have higher densities than branched-chain polymers.
E)Heteroatoms in polymers can drastically change the properties of a hydrocarbon polymer.

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
46
For each of the numbered bonds in the figure below, identify whether the bond is cis, trans, or neither cis nor trans. 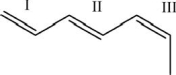
A)I-cis; II-trans; III-cis
B)I-trans; II-cis; III-trans
C)I-neither; II-trans; III-cis
D)I-neither; II-cis; III-trans
E)I-neither; II-cis; II-cis

A)I-cis; II-trans; III-cis
B)I-trans; II-cis; III-trans
C)I-neither; II-trans; III-cis
D)I-neither; II-cis; III-trans
E)I-neither; II-cis; II-cis

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
47
Where is the carbon-carbon double bond in the hydrocarbon shown? 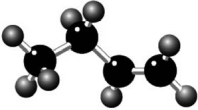
A)on the left
B)in the middle
C)on the right
D)on each bond
E)spread over all three bonds because of resonance

A)on the left
B)in the middle
C)on the right
D)on each bond
E)spread over all three bonds because of resonance

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
48
Delocalized bonding in aromatic hydrocarbons is possible because of
A)the fact that electrons can move very quickly from one resonance form to another.
B)adjacent 2pz orbitals each having one electron.
C)Wade's rules.
D)double-headed arrows.
E)Cooper pairs.
A)the fact that electrons can move very quickly from one resonance form to another.
B)adjacent 2pz orbitals each having one electron.
C)Wade's rules.
D)double-headed arrows.
E)Cooper pairs.

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
49
Does this molecule have both cis and trans isomers? 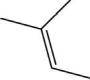
A)Yes, this is the cis isomer.
B)Yes, this is the trans isomer.
C)No, it has only the cis isomer.
D)No, it has only the trans isomer.
E)No, this molecule does not have cis-trans isomerism.

A)Yes, this is the cis isomer.
B)Yes, this is the trans isomer.
C)No, it has only the cis isomer.
D)No, it has only the trans isomer.
E)No, this molecule does not have cis-trans isomerism.

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
50
Name the compound.Note: Double and/or triple bonds are not shown. 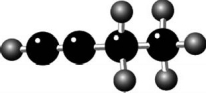
A)1-butene
B)1-butyne
C)2-butene
D)2-butyne
E)butane

A)1-butene
B)1-butyne
C)2-butene
D)2-butyne
E)butane

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
51
How many structural constitutional) isomers are there for propylene, and why?
A)two, because of resonance
B)two, because of geometrical isomerism
C)two, because the double bond can be in either one of two locations
D)none, because the two possible skeletal structures can be superimposed
E)none, because double bonds always occur on the right in alkenes
A)two, because of resonance
B)two, because of geometrical isomerism
C)two, because the double bond can be in either one of two locations
D)none, because the two possible skeletal structures can be superimposed
E)none, because double bonds always occur on the right in alkenes

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
52
Is cyclooctatetraene considered aromatic? The most stable structure at room temperature is shown. 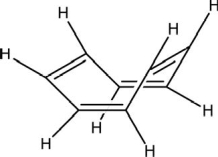
A)Yes, because there are alternating single and double carbon-carbon bonds.
B)Yes, because the electrons in the bonds are delocalized over the ring structure.
C)Yes, there is an even number of electrons involved in bonding.
D)No, the electrons in the bonds are not delocalized over the ring structure.
E)No, the carbons are not sp2 hybridized.

A)Yes, because there are alternating single and double carbon-carbon bonds.
B)Yes, because the electrons in the bonds are delocalized over the ring structure.
C)Yes, there is an even number of electrons involved in bonding.
D)No, the electrons in the bonds are not delocalized over the ring structure.
E)No, the carbons are not sp2 hybridized.

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following is/are true regarding aromatic hydrocarbons?
I.They are particularly stable because of delocalized bonding.
II.They are particularly stable because of their covalent network bonding.
III.They are particularly unstable, as evidenced by their tendency to evaporate.
A)I only
B)II only
C)III only
D)I and II only
E)I and III only
I.They are particularly stable because of delocalized bonding.
II.They are particularly stable because of their covalent network bonding.
III.They are particularly unstable, as evidenced by their tendency to evaporate.
A)I only
B)II only
C)III only
D)I and II only
E)I and III only

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following is NOT a difference between cyclohexane and benzene?
A)Cyclohexane is puckered, but benzene is planar.
B)Cyclohexane carbons are sp3 hybridized, but benzene carbons are sp2 hybridized.
C)Cyclohexane is not aromatic, but benzene is.
D)Cyclohexane has 12 hydrogen atoms in its structure, but benzene has 6 hydrogen atoms in its structure.
E)Cyclohexane is a combustible hydrocarbon, but benzene is not combustible due to aromaticity.
A)Cyclohexane is puckered, but benzene is planar.
B)Cyclohexane carbons are sp3 hybridized, but benzene carbons are sp2 hybridized.
C)Cyclohexane is not aromatic, but benzene is.
D)Cyclohexane has 12 hydrogen atoms in its structure, but benzene has 6 hydrogen atoms in its structure.
E)Cyclohexane is a combustible hydrocarbon, but benzene is not combustible due to aromaticity.

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
55
Consider the 1,2-dichloro derivative of ethylene in which one of the hydrogen atoms on each of the two carbon atoms in ethylene is substituted with a chlorine atom, giving the structural formula CHClCHCl.Which of the following is true?
A)The trans isomer is more polar.
B)The cis isomer is more polar.
C)Neither cis nor trans isomers are polar.
D)Both cis and trans isomers are equally polar.
E)There are no isomers for this molecule.
A)The trans isomer is more polar.
B)The cis isomer is more polar.
C)Neither cis nor trans isomers are polar.
D)Both cis and trans isomers are equally polar.
E)There are no isomers for this molecule.

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
56
A homopolymer is a polymer in which
A)each polymer has the same mass.
B)each polymer has the same mass and structure.
C)the monomers are distributed uniformly throughout the polymer.
D)the polymers are distributed uniformly throughout the solution.
E)there is only one type of monomer unit.
A)each polymer has the same mass.
B)each polymer has the same mass and structure.
C)the monomers are distributed uniformly throughout the polymer.
D)the polymers are distributed uniformly throughout the solution.
E)there is only one type of monomer unit.

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
57
Name the compound. 
A)trans-3-hexene
B)cis-3-hexene
C)trans-4-hexene
D)cis-4-hexene
E)1,2-diethylethylene

A)trans-3-hexene
B)cis-3-hexene
C)trans-4-hexene
D)cis-4-hexene
E)1,2-diethylethylene

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following pairs show constitutional isomers?
I.
II.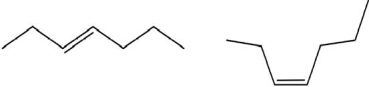
III.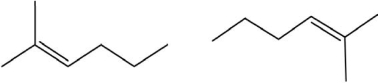
IV.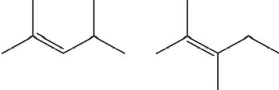
A)I
B)II
C)I and III
D)II and III
E)IV
I.

II.

III.
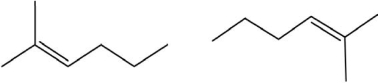
IV.

A)I
B)II
C)I and III
D)II and III
E)IV

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
59
Name the compound. 
A)cis-3-octene
B)trans-3-octene
C)cis-5-octene
D)trans-5-octene
E)1-ethyl-2-butyl ethylene

A)cis-3-octene
B)trans-3-octene
C)cis-5-octene
D)trans-5-octene
E)1-ethyl-2-butyl ethylene

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
60
Naphthalene, a polyaromatic hydrocarbon, is used for mothballs.Its molecular formula is C10H8.How many fused aromatic rings does naphthalene have in its molecular structure?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
61
The Aldrich Chemical Company catalogue lists the three isomers of xylene as having boiling points of 143-145 C, 138-13911eed930_22f1_bf38_a0ba_a9cce94f9837_TB6562_11C, and 13811eed930_22f1_bf38_a0ba_a9cce94f9837_TB6562_11Which isomer has the highest boiling point, and why?
C, 138-13911eed930_22f1_bf38_a0ba_a9cce94f9837_TB6562_11C, and 13811eed930_22f1_bf38_a0ba_a9cce94f9837_TB6562_11Which isomer has the highest boiling point, and why?
A)o-xylene (1,2-dimethylbenzene) because of its polarity
B)p-xylene (1,4-dimethylbenzene) because of its polarity
C)p-xylene (1,4-dimethylbenzene) because of its para-normalcy
D)m-xylene (1,3-dimethylbenzene) because of its C2 symmetry
E)o,m,p-xylene( 1,2,3,4-tetramethylbenzene) because of its molar mass
 C, 138-13911eed930_22f1_bf38_a0ba_a9cce94f9837_TB6562_11C, and 13811eed930_22f1_bf38_a0ba_a9cce94f9837_TB6562_11Which isomer has the highest boiling point, and why?
C, 138-13911eed930_22f1_bf38_a0ba_a9cce94f9837_TB6562_11C, and 13811eed930_22f1_bf38_a0ba_a9cce94f9837_TB6562_11Which isomer has the highest boiling point, and why?A)o-xylene (1,2-dimethylbenzene) because of its polarity
B)p-xylene (1,4-dimethylbenzene) because of its polarity
C)p-xylene (1,4-dimethylbenzene) because of its para-normalcy
D)m-xylene (1,3-dimethylbenzene) because of its C2 symmetry
E)o,m,p-xylene( 1,2,3,4-tetramethylbenzene) because of its molar mass

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
62
The C - O - H bonding arrangement is called the ______functional group.
A)alcohol
B)ester
C)amine
D)ether
E)carbonyl
A)alcohol
B)ester
C)amine
D)ether
E)carbonyl

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
63
How many structural isomers does xylenol, (CH3)2C6H3OH, have?
A)None-there is only 1 molecule.
B)2
C)4
D)6
E)8
A)None-there is only 1 molecule.
B)2
C)4
D)6
E)8

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
64
What functional group is found in the following molecule? 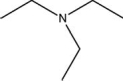
A)amine
B)amide
C)ammonia
D)ammonium
E)amonyl

A)amine
B)amide
C)ammonia
D)ammonium
E)amonyl

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
65
How many structural isomers does xylene, C6H4(CH3)2, have?
A)None-there is only 1 structure.
B)2
C)3
D)4
E)5
A)None-there is only 1 structure.
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
66
What is another name for methylbenzene?
A)toluene
B)xylene
C)naphthalene
D)benzoyl methane
E)1-methyl-2,4,6-cyclohexene
A)toluene
B)xylene
C)naphthalene
D)benzoyl methane
E)1-methyl-2,4,6-cyclohexene

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following is an amine functional group?
A)(-COOH)
B)(-OH)
C)(-CN)
D)(-NH2)
E)(-CONH2)
A)(-COOH)
B)(-OH)
C)(-CN)
D)(-NH2)
E)(-CONH2)

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following is NOT a product when bacteria of the genus Methanosarcina act on amines? If all the compounds listed are products of the reaction, select E.
A)ammonia, NH3
B)methane, CH4
C)hydrogen cyanide, HCN
D)carbon dioxide, CO2
E)All of these compounds are products of the reaction.
A)ammonia, NH3
B)methane, CH4
C)hydrogen cyanide, HCN
D)carbon dioxide, CO2
E)All of these compounds are products of the reaction.

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
69
Methane used for fuel and commonly called natural gas is odorless, but for safety it has a small quantity of foul-smelling methylmercaptan CH3SH) added.The "mercaptan" functional group is most similar to which of the following?
A)carboxylic acid
B)amine
C)ether
D)aldehyde
E)alcohol
A)carboxylic acid
B)amine
C)ether
D)aldehyde
E)alcohol

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
70
What functional group is found in oil of peppermint? 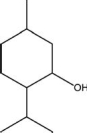
A)carboxylic acid
B)aldehyde
C)alcohol
D)carbonyl
E)hydroxyl

A)carboxylic acid
B)aldehyde
C)alcohol
D)carbonyl
E)hydroxyl

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
71
The molecular structure of thalidomide is shown below.Nitrogen II could be classified as a(n)_______ amine. 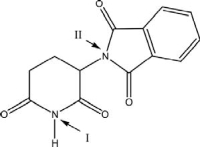
A)aromatic
B)auxiliary
C)primary
D)secondary
E)tertiary

A)aromatic
B)auxiliary
C)primary
D)secondary
E)tertiary

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
72
What type of amine is adrenaline, which is depicted below? 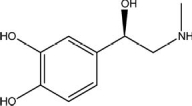
A)aromatic
B)auxiliary
C)primary
D)secondary
E)tertiary

A)aromatic
B)auxiliary
C)primary
D)secondary
E)tertiary

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
73
The characteristic "fishy" odor comes from methylamine (CH3NH2), which is similar to ammonia (NH3) in structure and also in its ability to react with acids as a weak base.Why would the acid form of methylamine NOT have as strong an odor as methylamine?
A)because it would decompose to methane and ammonia
B)because it would not be volatile, being a polyatomic ion
C)because it would melt in the acid
D)because it would decompose to carbon, hydrogen, and nitrogen
E)because it would have a different three-dimensional structure
A)because it would decompose to methane and ammonia
B)because it would not be volatile, being a polyatomic ion
C)because it would melt in the acid
D)because it would decompose to carbon, hydrogen, and nitrogen
E)because it would have a different three-dimensional structure

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
74
How many structural isomers does toluene, C6H5CH3, have?
A)None-there is only 1 molecule.
B)2
C)3
D)4
E)5
A)None-there is only 1 molecule.
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
75
The monomer for polystyrene is shown below.Which of the bonds is involved in the polymerization process? 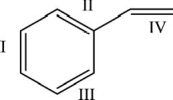
A)I only
B)II only
C)III only
D)IV only
E)Bonds I-IV are all involved in polymerization.

A)I only
B)II only
C)III only
D)IV only
E)Bonds I-IV are all involved in polymerization.

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
76
Gasoline additives such as ethanol are employed to
A)decrease odor.
B)promote complete combustion.
C)decrease octane rating.
D)make the fuel less volatile.
E)increase fuel value.
A)decrease odor.
B)promote complete combustion.
C)decrease octane rating.
D)make the fuel less volatile.
E)increase fuel value.

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following molecules, all of which contain at least one alcohol functional group, would you predict is the least soluble in water?
A)vitamin C,
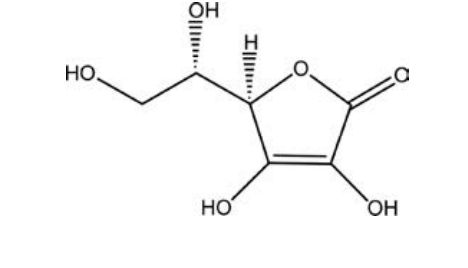
B)glycerol,
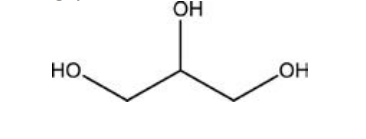
C) -D-glucose,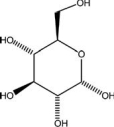
D)cholesterol,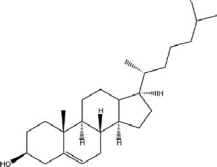
E)adrenaline,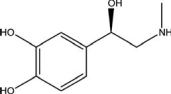
A)vitamin C,

B)glycerol,

C) -D-glucose,

D)cholesterol,

E)adrenaline,


Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
78
The molecular structure of caffeine is shown below.The nitrogen atoms could be classified as _____amines. 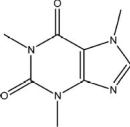
A)primary
B)secondary
C)tertiary
D)auxiliary
E)aromatic

A)primary
B)secondary
C)tertiary
D)auxiliary
E)aromatic

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following class of compounds is NOT generally used in gasoline?
A)alcohols
B)ethers
C)carboxylic acids
D)alkanes
E)thiols
A)alcohols
B)ethers
C)carboxylic acids
D)alkanes
E)thiols

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck
80
The molecular structure of thalidomide is shown below.Nitrogen I could be classified as a(n)_____ amine. 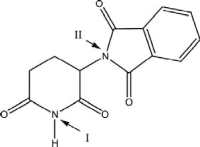
A)tertiary
B)secondary
C)primary
D)aromatic
E)auxiliary

A)tertiary
B)secondary
C)primary
D)aromatic
E)auxiliary

Unlock Deck
Unlock for access to all 145 flashcards in this deck.
Unlock Deck
k this deck



