Deck 7: Delocalized Electrons Aromaticity and the Reactions of Benzene
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/85
Play
Full screen (f)
Deck 7: Delocalized Electrons Aromaticity and the Reactions of Benzene
1
Which of the following is a benzylic cation? I.
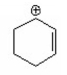
II.
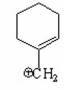
III.
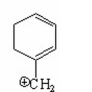
IV.
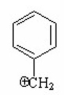
V.
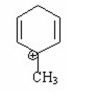
A) I
B) II
C) III
D) IV
E) V

II.

III.

IV.

V.

A) I
B) II
C) III
D) IV
E) V
IV
2
Which of the following does not have delocalized electrons?
A) allyl cation
B) benzyl anion
C) CO32-
D) benzyl cation
E) cyclohexyl radical
A) allyl cation
B) benzyl anion
C) CO32-
D) benzyl cation
E) cyclohexyl radical
cyclohexyl radical
3
What type of diene is represented by 1,5-octadiene?
A) conjugated diene
B) isolated diene
C) alkynyl diene
D) none of the above
A) conjugated diene
B) isolated diene
C) alkynyl diene
D) none of the above
isolated diene
4
Which has correctly drawn arrows?
A)
B)
C)
D)
E)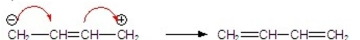
A)

B)

C)

D)

E)
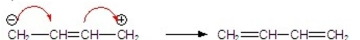

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
5
What type of diene is represented by 2,4-hexadiene?
A) conjugated diene
B) isolated diene
C) alkynyl diene
D) none of the above
A) conjugated diene
B) isolated diene
C) alkynyl diene
D) none of the above

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following statements is not correct about benzene?
A) All of the carbon atoms are sp hybridized.
B) It has delocalized electrons.
C) The carbon-carbon bonds are all the same length.
D) The carbon-hydrogen bonds are all the same length.
E) All twelve atoms lie in the same plane.
A) All of the carbon atoms are sp hybridized.
B) It has delocalized electrons.
C) The carbon-carbon bonds are all the same length.
D) The carbon-hydrogen bonds are all the same length.
E) All twelve atoms lie in the same plane.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is an allylic cation? I.
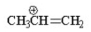
II.
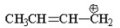
III.

IV.
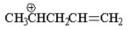
V.
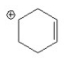
A) I
B) II
C) III
D) IV
E) V
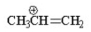
II.
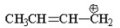
III.

IV.
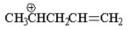
V.

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following compounds has the shortest bond between its two middle carbons?
A)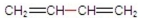
B)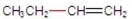
C)
D)
E)
A)
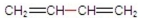
B)
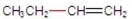
C)

D)

E)


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following is the most stable?
A)
B)
C)
D)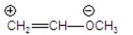
E)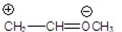
A)
B)
C)

D)
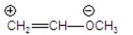
E)


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following pairs are resonance contributors? I. and
II.
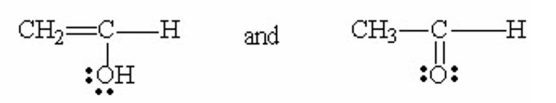
III.
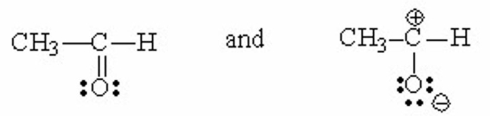
IV.
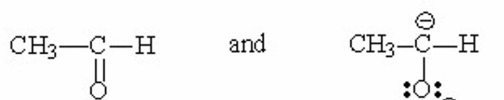
V.
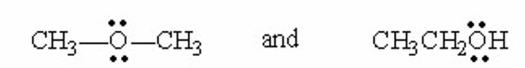
A) I
B) II
C) III
D) IV
E) V

II.
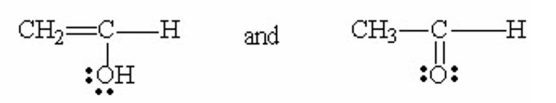
III.
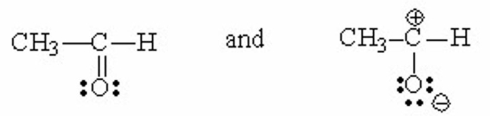
IV.
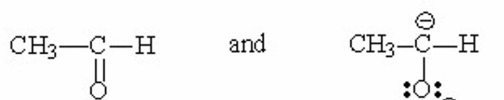
V.
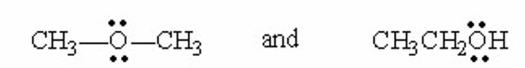
A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is the most stable carbocation? I.
II.
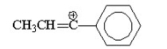
III.
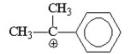
IV.
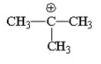
V.
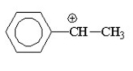
A) I
B) II
C) III
D) IV
E) V
II.

III.

IV.

V.

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following statements about resonance contributors and resonance hybrids is not correct?
A) The fewer nearly equivalent resonance contributors, the greater the resonance energy.
B) A resonance hybrid is more stable than the predicted stability of any of its resonance contributors.
C) The greater the number of relatively stable resonance contributors, the greater the resonance energy.
D) The greater the predicted stability of a resonance contributor, the more it contributes to the resonance hybrid.
E) none of the above
A) The fewer nearly equivalent resonance contributors, the greater the resonance energy.
B) A resonance hybrid is more stable than the predicted stability of any of its resonance contributors.
C) The greater the number of relatively stable resonance contributors, the greater the resonance energy.
D) The greater the predicted stability of a resonance contributor, the more it contributes to the resonance hybrid.
E) none of the above

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following statements about benzene is correct?
A) All of the carbon atoms are sp3 hybridized.
B) It has no delocalized electrons.
C) The carbon-carbon bonds are longer than the carbon-carbon bond in ethane.
D) It is a planar molecule.
E) The carbon-hydrogen bonds are not the same length.
A) All of the carbon atoms are sp3 hybridized.
B) It has no delocalized electrons.
C) The carbon-carbon bonds are longer than the carbon-carbon bond in ethane.
D) It is a planar molecule.
E) The carbon-hydrogen bonds are not the same length.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is the most stable diene? I.
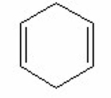
II.

III.
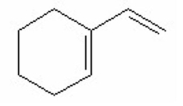
IV.
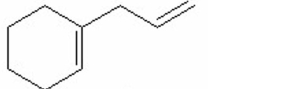
V.
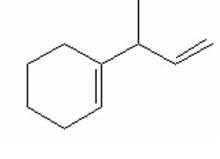
A) I
B) II
C) III
D) IV
E) V

II.

III.

IV.

V.

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following pairs are resonance contributors? I.
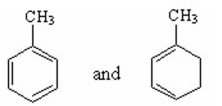
II.
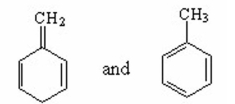
III.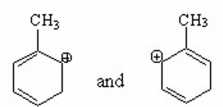
IV.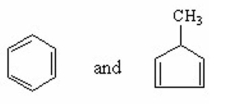
V.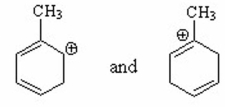
A) I
B) II
C) III
D) IV
E) V

II.

III.

IV.

V.
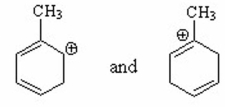
A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is a resonance hybrid?
A)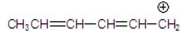
B)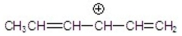
C)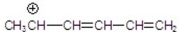
D)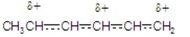
E) A,B, and C.
A)
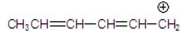
B)
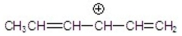
C)
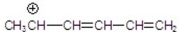
D)
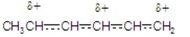
E) A,B, and C.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following is not a resonance contributor of the species shown below? 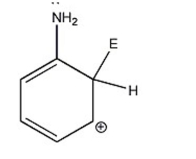
I.
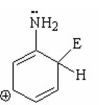
II.
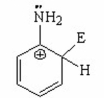
III.
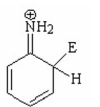
IV.
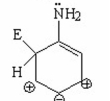
A) I
B) II
C) III
D) IV

I.

II.

III.

IV.

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following compounds contains the longest carbon-carbon single bond?
A) propene
B) 1,3-butadiyne
C) 1,3-butadiene
D) propyne
E) ethane
A) propene
B) 1,3-butadiyne
C) 1,3-butadiene
D) propyne
E) ethane

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
19
Due to electron delocalization, one would predict that the carbon-oxygen bond in CH3CONH2.
A) is nonpolar
B) has more double bond character than the carbon-oxygen bond of (CH3)2CO
C) is longer than the carbon-oxygen bond of (CH3)2O
D) is longer than the carbon-oxygen bond of (CH3)2CO
E) is formed by overlapping sp3 orbitals
A) is nonpolar
B) has more double bond character than the carbon-oxygen bond of (CH3)2CO
C) is longer than the carbon-oxygen bond of (CH3)2O
D) is longer than the carbon-oxygen bond of (CH3)2CO
E) is formed by overlapping sp3 orbitals

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
20
The delocalized cloud of π electrons in benzene is formed by the overlap of 6 ________ orbitals.
A) s
B) p
C) sp
D) sp2
E) sp3
A) s
B) p
C) sp
D) sp2
E) sp3

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
21
What are the major products of the following reaction?
A)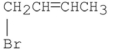
B)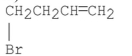
C)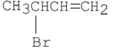
D) A and B
E) A and C
A)
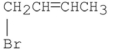
B)
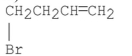
C)
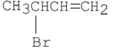
D) A and B
E) A and C

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
22
What are the major products of the following reaction?
A)
B)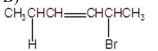
C)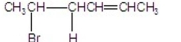
D)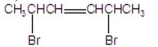
E)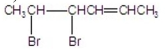
A)

B)
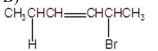
C)
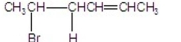
D)
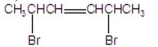
E)


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
23
What are the major products of the following reaction? 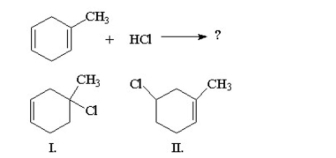
A) I
B) II
C) I and II with more II and I
D) neither I nor II
E) equal amounts of I and II

A) I
B) II
C) I and II with more II and I
D) neither I nor II
E) equal amounts of I and II

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
24
What is the major product of the following reaction?
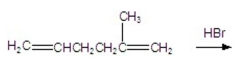
A)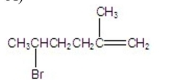
B)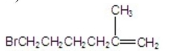
C)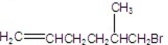
D)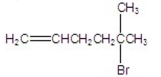
E)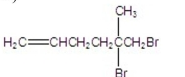

A)

B)
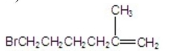
C)
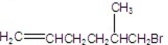
D)
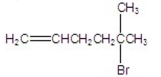
E)


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
25
What is the major product of the following reaction?
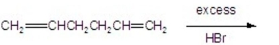
A)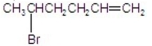
B)
C)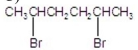
D)
E)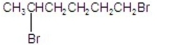
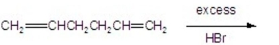
A)
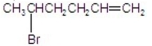
B)
C)
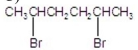
D)
E)

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
26
What is the major product of the following reaction?
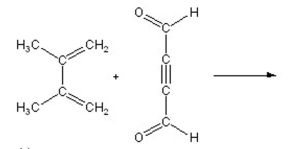
A)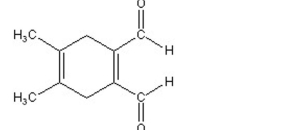
B)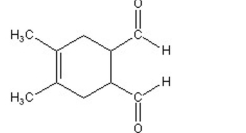
C)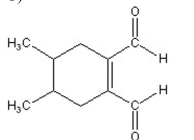
D)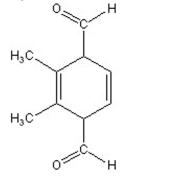
E) no reaction

A)

B)

C)

D)

E) no reaction

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
27
What is the major product of the following reaction? 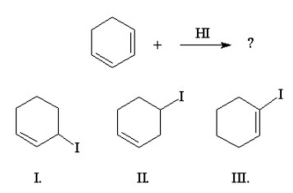
A) I
B) I and II
C) I and III
D) II
E) III

A) I
B) I and II
C) I and III
D) II
E) III

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
28
Draw the major product of the following reaction. 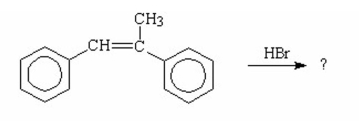
I.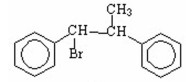
II.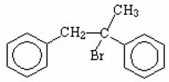
II.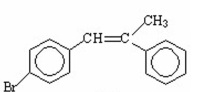
A) I
B) I and III
C) II
D) II and III
E) III

I.

II.

II.

A) I
B) I and III
C) II
D) II and III
E) III

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following is the strongest acid? I.
II.
III.
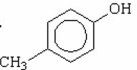
IV.
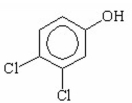
V.
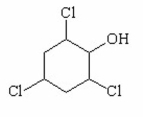
A) I
B) II
C) III
D) IV
E) V

II.

III.

IV.

V.

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
30
Which is the most stable carbocation?
A)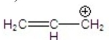
B)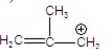
C)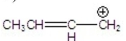
D)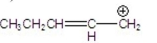
E)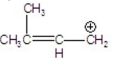
A)

B)

C)
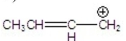
D)
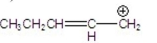
E)


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
31
What are the major products of the following reaction?
I.
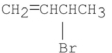
II.
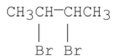
III.
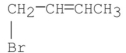
A) I only
B) II only
C) III only
D) I and II
E) I and III
I.

II.
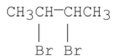
III.
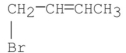
A) I only
B) II only
C) III only
D) I and II
E) I and III

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
32
What compound results from the 1,4-addition of one equivalent of HBr to 1,3-butadiene?
A) 1-bromo-1-butene
B) 2-bromo-2-butene
C) 4-bromo-1-butene
D) 3-bromo-1-butene
E) 1-bromo-2-butene
A) 1-bromo-1-butene
B) 2-bromo-2-butene
C) 4-bromo-1-butene
D) 3-bromo-1-butene
E) 1-bromo-2-butene

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
33
What is the major product of the following reaction? 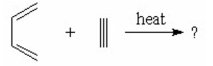
I.
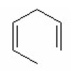
II.
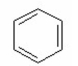
III.
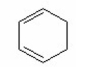
IV.
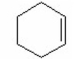
V.
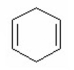
A) I
B) II
C) III
D) IV
E) V
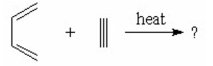
I.

II.

III.

IV.

V.

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
34
What is the major product of the following reaction?
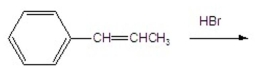
A)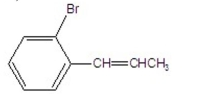
B)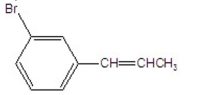
C)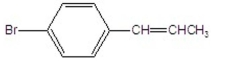
D)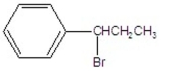
E)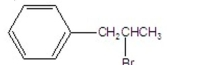

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following ammonium ions is the strongest acid?
A)C6H5NH3+
B)C6H5CH2NH3+
C) (CH3)2CHNH3+
D)CH3CH2NH3+
E)CH3NH3+
A)C6H5NH3+
B)C6H5CH2NH3+
C) (CH3)2CHNH3+
D)CH3CH2NH3+
E)CH3NH3+

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
36
When 1,3-butadiene reacts with CH2 = CHCN , which of the following terms describes the product?
A) a mixture of two diastereomers
B) a single compound
C) a racemic mixture
D) optically active
E) a mixture of bicyclic compounds
A) a mixture of two diastereomers
B) a single compound
C) a racemic mixture
D) optically active
E) a mixture of bicyclic compounds

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following is the weakest base?
A) phenolate ion (C6H5O-)
B) ethoxide ion (CH3CH2O-)
C) hydroxide ion (HO-)
D) acetate ion (CH3CO2-)
E) methoxide ion (CH3O-)
A) phenolate ion (C6H5O-)
B) ethoxide ion (CH3CH2O-)
C) hydroxide ion (HO-)
D) acetate ion (CH3CO2-)
E) methoxide ion (CH3O-)

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following is a conjugated diene? I.
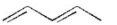
II.
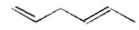
III.
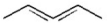
IV.
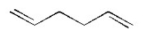
V.
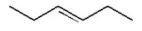
A) I
B) II
C) III
D) IV
E) V
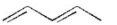
II.

III.

IV.

V.

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following is the strongest acid?
A)
B)
C)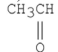
D)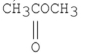
E)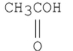
A)
B)
C)
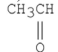
D)
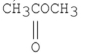
E)
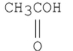

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following is an isolated diene? I.
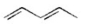
II.
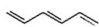
III.
11ee043e_be72_28fb_854b_9d8e1369c682_TB1829_11
IV.
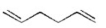
V.
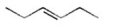
A) I
B) II
C) III
D) IV
E) V
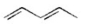
II.

III.
11ee043e_be72_28fb_854b_9d8e1369c682_TB1829_11
IV.

V.

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
41
Do the following represent different compounds or resonance contributors? 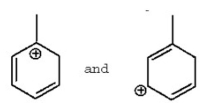


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
42
Aromatic molecules contain ________ π electrons.
A) no
B) an odd number of pairs of
C) an even number of pairs of
D) an odd number of
E) an even number of
A) no
B) an odd number of pairs of
C) an even number of pairs of
D) an odd number of
E) an even number of

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the oxygens in the acetate ion (CH3CO2-) has a greater negative charge? Explain
your answer.
your answer.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
44
Draw the resonance contributors for the following species: 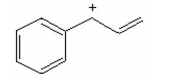


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following is aromatic? I.
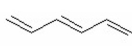
II.
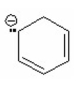
III.
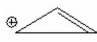
IV.
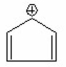
V.

A) I
B) II
C) III
D) IV
E) V

II.

III.

IV.

V.

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
46
Draw the other two resonance contributors for the following species: 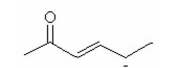


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
47
Draw the other two resonance contributors for the following species: 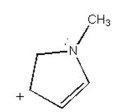


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
48
What is the hybridization and bond angle of each carbon in benzene?

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
49
What is the hybridization of the carbons labeled A, B, and C? 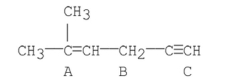


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
50
Draw the other two resonance contributors for the following species:
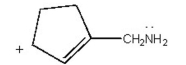


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
51
Draw the resonance contributors for the following species: 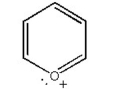


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following is an aromatic hydrocarbon? I.
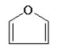
II.
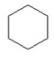
III.
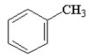
IV.
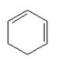
V.
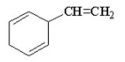
A) I
B) II
C) III
D) IV
E) V

II.

III.

IV.

V.

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
53
Draw the other two resonance contributors for the following species: 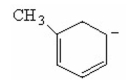


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
54
Mark the most electron-rich carbon in the compound below with an asterisk. CH3CH=CHNHCH3

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
55
Draw the other two resonance contributors for the following species: 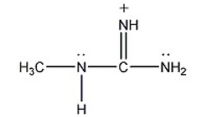


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following is aromatic?
A) the cyclopentadienyl cation
B) 1,3-cyclohexadiene
C) the cyclobutenyl anion
D) 1,3,5-hexatriene
E) the cycloheptatrienyl cation
A) the cyclopentadienyl cation
B) 1,3-cyclohexadiene
C) the cyclobutenyl anion
D) 1,3,5-hexatriene
E) the cycloheptatrienyl cation

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
57
Draw the other two resonance contributors for the following species: 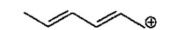


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following is aromatic? I.
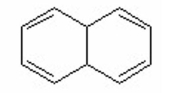
II.
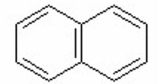
III.
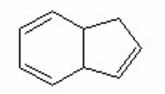
IV.
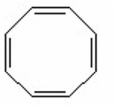
V.
A) I
B) II
C) III
D) IV
E) V

II.

III.

IV.

V.

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
59
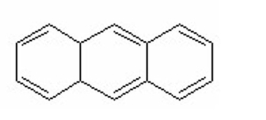
Which of the following is aromatic? 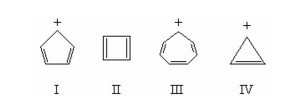
A) I and IV
B) I, III, and IV
C) III and IV
D) II

A) I and IV
B) I, III, and IV
C) III and IV
D) II

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
60
Draw the resonance contributor for the following carbocation: 


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
61
What is the major product of the following reaction? 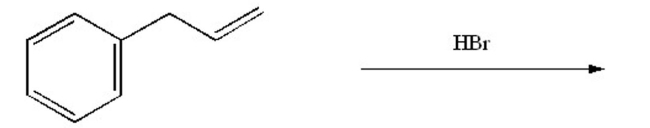
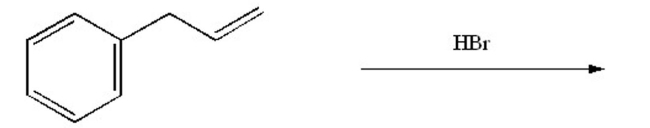

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following is the strongest acid? 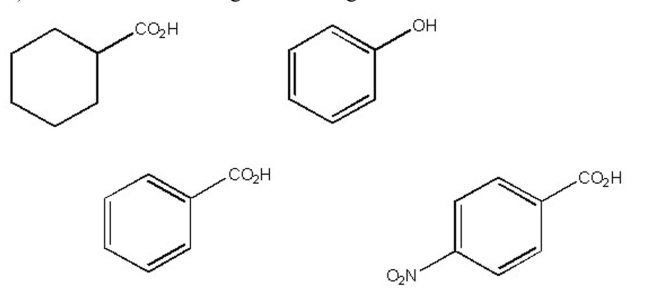


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
63
Draw the major product of the following reaction: 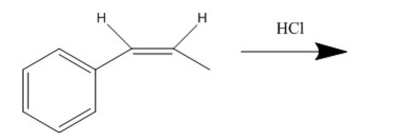


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
64
Why is the following compoound not aromatic? 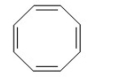


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
65
Draw the major product of the following reaction: 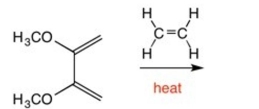


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
66
Draw the arrows to go from one resonance contributor to another. 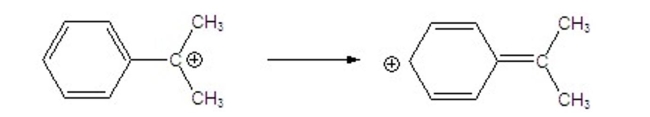


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
67
Draw the major product of the following reaction: 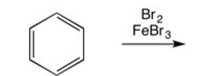


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
68
What is the product of the following Diels-Alder reaction? 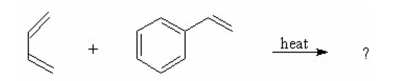


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
69
List the criteria that compounds must meet in order to be aromatic.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following carbocations is more stable? Explain. 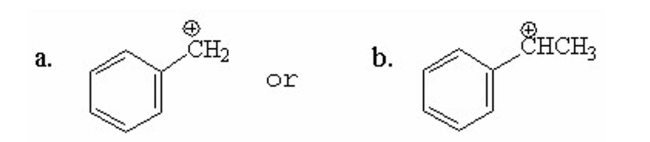
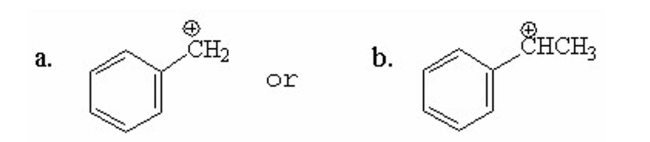

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
71
Is the following molecule aromatic? Explain. 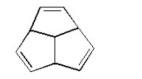


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
72
What is the major product of the following reaction? 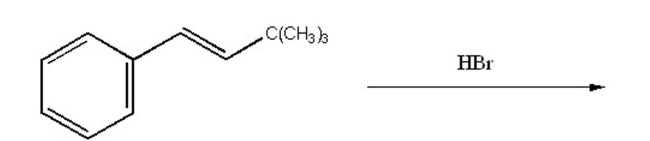


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
73
Draw the major product of the following reaction: 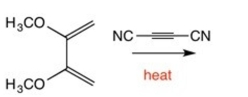


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
74
Why is benzene's heat of hydrogenation 36 kcal/mol less than three times that of cyclohexene?

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
75
What diene and dienophile will react to form the product shown below? 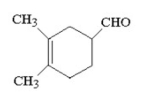


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
76
How could the following compound be synthesized using a Diels-Alder reaction? 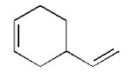


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
77
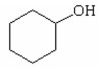
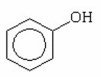
Why is phenol a stronger acid than cyclohexanol? 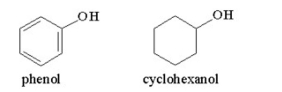


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
78
Draw the product that is formed from 1,4-addition of HBr to the following diene: 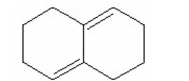


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
79
Draw the resonance contributors for the following species: 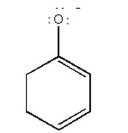


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
80
Draw the product that is formed from 1,2-addition of HBr to the following diene: 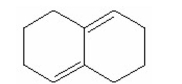


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck



