Deck 8: Substitution Reactions of Alkyl Halides
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/92
Play
Full screen (f)
Deck 8: Substitution Reactions of Alkyl Halides
1
Which of the following SN2 reactions is the slowest?
A)
B)
C)
D)
A)
B)
C)
D)
2
Which of the following is a secondary alkyl halide?
A)CH3Br
B) (CH3)3CBr
C) (CH3)2CHBr
D)(CH3)2CHCH2Br
A)CH3Br
B) (CH3)3CBr
C) (CH3)2CHBr
D)(CH3)2CHCH2Br
(CH3)2CHBr
3
When (S)-2-bromobutane undergoes an SN2 , the product is the compound shown below. What is the configuration of the product? 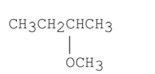
A) S only
B) R only
C) both R and S with more R than S
D) both R and S with more S than R
E) equal amounts of R and S
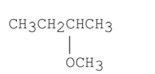
A) S only
B) R only
C) both R and S with more R than S
D) both R and S with more S than R
E) equal amounts of R and S
R only
4
Which of the following alkyl halides reacts the fastest in an SN2 reaction?
A) 1-chloropropane
B) 1-bromopropane
C) 1-fluoropropane
D) 1-iodopropane
A) 1-chloropropane
B) 1-bromopropane
C) 1-fluoropropane
D) 1-iodopropane

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
5
What is the nucleophile in the following reaction? 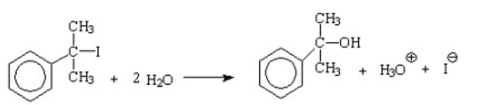
I.
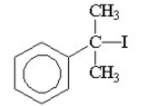
II.
III.
IV.
V.
A) I
B) II
C) III
D) IV
E) V

I.

II.
III.
IV.
V.
A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
6
-How does doubling the concentration of both the alkyl halide and the nucleophile in the above SN2 reaction affect the rate of the reaction?
A) no change
B) doubles the rate
C) triples the rate
D) quadruples the rate
E) rate is halved

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following alkyl halides undergoes an SN2 reaction most rapidly?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following SN2 reactions is the slowest?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)


Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following SN2 reactions is the fastest?
A)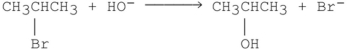
B)
C)
D)
E)
A)

B)
C)

D)
E)

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is not a nucleophile?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
11
The specific rotation of was converted into the corresponding alcohol via a SN2 eaction. What is the specific rotation of the product?
A) -42.3
B) between 0 and -42.3
C) between +42.3 and 0
D) +42.3
A) -42.3
B) between 0 and -42.3
C) between +42.3 and 0
D) +42.3

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following alkyl halides is the least reactive in an SN2 reaction?
A)CH3CH2Cl
B)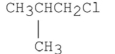
C)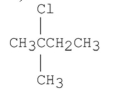
D)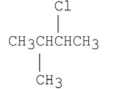
E)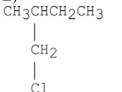
A)CH3CH2Cl
B)
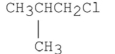
C)

D)

E)


Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
13
The specific rotation of (R)-sec-butyl alcohol is -13.52. A pure sample of (R)-sec-butyl bromide was converted to sec-butyl alcohol via an SN2 reaction. What is the specific rotation of
The product, assuming 100% yield?
A) -13.52
B) between 0 and -13.52
C) between 0 and +13.52
D) +13.52
E) zero
The product, assuming 100% yield?
A) -13.52
B) between 0 and -13.52
C) between 0 and +13.52
D) +13.52
E) zero

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is not a nucleophile?
A) NH3
B) NH2CH3
C)
D) CH3CH2+
A) NH3
B) NH2CH3
C)
D) CH3CH2+

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
15
What are the substitution products of the following reaction?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
16
-How does doubling the concentration of the alkyl halide in the above SN2 reaction affect the rate of the reaction?
A) no change
B) doubles the rate
C) triples the rate
D) quadruples the rate
E) rate is halved

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
17
What product results from the SN2 reaction between (R)-2-chloropentane and methoxide ion?
A) (R)-2-methoxypentane
B) (S)-2-methoxypentane
C) racemic 2-methoxypentane
D) 1-methoxypentane
E) 3-methoxypentane
A) (R)-2-methoxypentane
B) (S)-2-methoxypentane
C) racemic 2-methoxypentane
D) 1-methoxypentane
E) 3-methoxypentane

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following alkyl halides reacts the fastest in an SN2 reaction?
A) 2-chloro-2-methylpropane
B) 2-chlorobutane
C) 1-chlorobutane
D) chloromethane
A) 2-chloro-2-methylpropane
B) 2-chlorobutane
C) 1-chlorobutane
D) chloromethane

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following SN2 reactions is the fastest?
A)
B)
C)
D)
A)
B)
C)
D)

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following is the best nucleophile in an SN2 reaction?
A) isopropoxide ion
B) tert-butoxide ion
C) ethoxide ion
D) tert-pentoxide ion
A) isopropoxide ion
B) tert-butoxide ion
C) ethoxide ion
D) tert-pentoxide ion

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
21
Which alkyl halide undergoes an SN2 reaction most rapidly?
A) 1-iodo-3-methylpentane
B) 2 -iodopentane
C) 2-iodo-2-methylpentane
D) 3 -iodopentane
E) 1 -iodo-2,2-dimethylpentane
A) 1-iodo-3-methylpentane
B) 2 -iodopentane
C) 2-iodo-2-methylpentane
D) 3 -iodopentane
E) 1 -iodo-2,2-dimethylpentane

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following is the best leaving group?
A) HO-
B) F-
C) Cl-
D) Br-
E) I-
A) HO-
B) F-
C) Cl-
D) Br-
E) I-

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following species is the least nucleophilic?
A) (CH3)3CO-
B) H2O
C) (CH3)3N
D) HO-
E) CN-
A) (CH3)3CO-
B) H2O
C) (CH3)3N
D) HO-
E) CN-

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following alkyl halides reacts the fastest in an SN1 reaction?
A)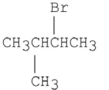
B)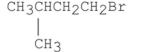
C)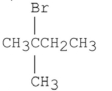
D)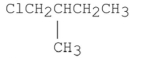
E)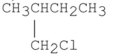
A)

B)
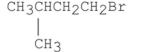
C)

D)
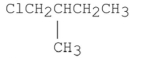
E)
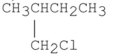

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
25
-How does doubling the concentration of the alkyl halide affect the rate of the above SN1 reaction?
A) no change
B) doubles the rate
C) triples the rate
D) quadruples the rate
E) rate is halved

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
26
When (S)-1-bromo-1-phenylethane undergoes an SN2 reaction with methanethiol (CH3SH) the product of the reaction is the compound shown below. What is its configuration?
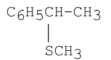
A) S only
B) R only
C) both R and S with slightly more S than R
D) both R and S with slightly more R than S
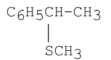
A) S only
B) R only
C) both R and S with slightly more S than R
D) both R and S with slightly more R than S

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
27
SN2 reactions usually proceed with ________.
A) equal amounts of inversion and retention at the asymmetric center undergoing substitution
B) slightly more inversion than retention at the asymmetric center undergoing substitution
C) slightly more retention than inversion at the asymmetric center undergoing substitution
D) complete inversion at the asymmetric center undergoing substitution
E) complete retention at the asymmetric center undergoing substitution
A) equal amounts of inversion and retention at the asymmetric center undergoing substitution
B) slightly more inversion than retention at the asymmetric center undergoing substitution
C) slightly more retention than inversion at the asymmetric center undergoing substitution
D) complete inversion at the asymmetric center undergoing substitution
E) complete retention at the asymmetric center undergoing substitution

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following statements concerning SN2 reactions of alkyl halides is not correct?
A) The rate of reaction depends on the concentration of the nucleophile.
B) The rate of reaction depends on the concentration of the alkyl halide.
C) The rate of reaction of a particular alkyl bromide depends on the steric accessibility of the carbon of the C-Br bond.
D) All alkyl iodides react more rapidly than all alkyl chlorides.
E) The rate of reaction depends on the relative nucleophilicity of the nucleophile.
A) The rate of reaction depends on the concentration of the nucleophile.
B) The rate of reaction depends on the concentration of the alkyl halide.
C) The rate of reaction of a particular alkyl bromide depends on the steric accessibility of the carbon of the C-Br bond.
D) All alkyl iodides react more rapidly than all alkyl chlorides.
E) The rate of reaction depends on the relative nucleophilicity of the nucleophile.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
29
The SN1 reaction of (CH3)3)CBr with water has ________ steps, ________ transition states, and ________ intermediates.
A) 2; 2; 2
B) 2; 2; 3
C) 2; 3; 2
D) 3; 2; 3
E) 3; 3; 2
A) 2; 2; 2
B) 2; 2; 3
C) 2; 3; 2
D) 3; 2; 3
E) 3; 3; 2

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
30
Which alkyl halide undergoes an SN2 reaction most rapidly?
A)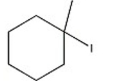
B)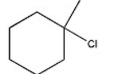
C)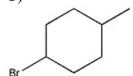
D)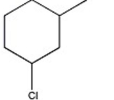
E)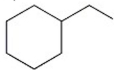
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following describes the relative nucleophilicities of methoxide and tert- butoxide?
A) These alkoxides have the same nucleophilicity because the negative charge in both is on an oxygen atom.
B) Methoxide is more nucleophilic because the nucleophilicity of tert-butoxide is decreased by steric effects.
C) tert-Butoxide is more nucleophilic because it contains three methyl groups which increase the charge on its oxygen by donating electron density.
D) tert-Butoxide is more nucleophilic because it is more basic.
E) none of the above
A) These alkoxides have the same nucleophilicity because the negative charge in both is on an oxygen atom.
B) Methoxide is more nucleophilic because the nucleophilicity of tert-butoxide is decreased by steric effects.
C) tert-Butoxide is more nucleophilic because it contains three methyl groups which increase the charge on its oxygen by donating electron density.
D) tert-Butoxide is more nucleophilic because it is more basic.
E) none of the above

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following factors has no effect on the rate of SN1 eactions?
A) the nature of the alkyl halide
B) the nature of the leaving group
C) the concentration of the alkyl halide
D) the concentration of the nucleophile
E) the value of the rate constant
A) the nature of the alkyl halide
B) the nature of the leaving group
C) the concentration of the alkyl halide
D) the concentration of the nucleophile
E) the value of the rate constant

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following compounds is the most reactive in a SN2 reaction?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following is the rate law for an SN1 reaction?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
35
-How does doubling the concentration of both the alkyl halide and the nucleophile affect the rate of the abov SN1 reaction?
A) no change
B) doubles the rate
C) triples the rate
D) quadruples the rate
E) rate is halved

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
36
-How does doubling the concentration of the nucleophile affect the rate of the above SN1 reaction?
A) no change
B) doubles the rate
C) triples the rate
D) quadruples the rate
E) rate is halved

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following alkyl halides reacts the fastest in an SN2 reaction?
A)
B)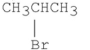
C)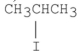
D)
E)
A)
B)
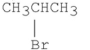
C)

D)
E)

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following alkyl halides reacts the fastest in a SN1 reaction?
A) 2-chloro-2-methylpropane
B) 2-chlorobutane
C) 1-chlorobutane
D) chloromethane
A) 2-chloro-2-methylpropane
B) 2-chlorobutane
C) 1-chlorobutane
D) chloromethane

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
39
Which alkyl halide undergoes a SN2 reaction most rapidly?
A)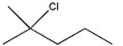
B)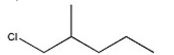
C)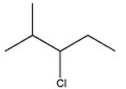
D)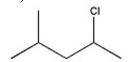
E)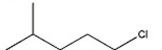
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
40
Which alkyl halide is the most reactive in an SN2 reaction?
A)
B)
C)
D)
E) All primary halides react at the same rate in SN2 reactions.
A)
B)
C)
D)
E) All primary halides react at the same rate in SN2 reactions.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
41
What is the major product of the following reaction? 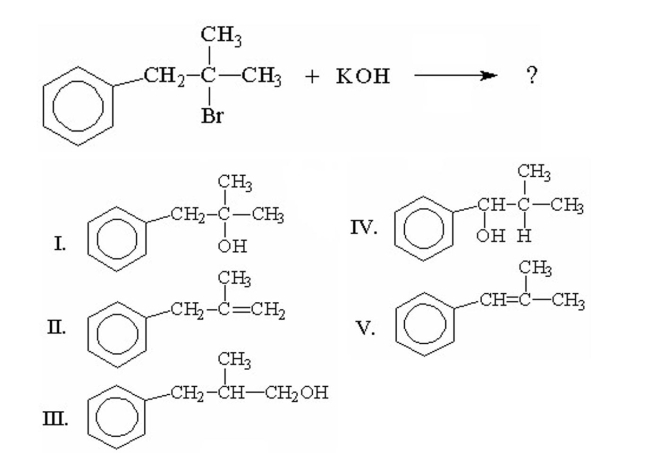
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following alkyl halides reacts the fastest in an E2 reaction?
A) 2-chloro-2-methylbutane
B) 1-chlorobutane
C) 1-chloro-2-methylbutane
D) 2-chlorobutane
E) chloromethane
A) 2-chloro-2-methylbutane
B) 1-chlorobutane
C) 1-chloro-2-methylbutane
D) 2-chlorobutane
E) chloromethane

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
43
What is the major product of the following E2 reaction?
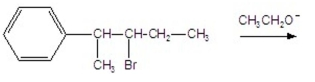
A)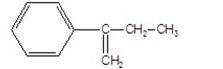
B)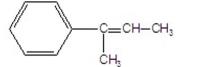
C)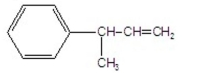
D)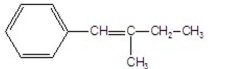
E)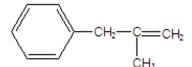

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following alkyl halides reacts the fastest in an E2 reaction?
A)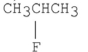
B)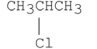
C)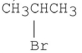
D)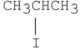
E)CH3I
A)
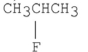
B)
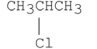
C)
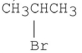
D)
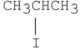
E)CH3I

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following alkyl chlorides forms only one alkene when it undergoes an elimination reaction?
A) 1-chloropentane
B) 2-chloropentane
C) 3-chloropentane
D) 1-chloro-2-methylbutane
E) 1-chloro-3-methylbutane
A) 1-chloropentane
B) 2-chloropentane
C) 3-chloropentane
D) 1-chloro-2-methylbutane
E) 1-chloro-3-methylbutane

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
46
What stereoisomer is the major product obtained from the following E2 reaction?
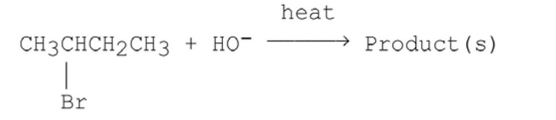
A)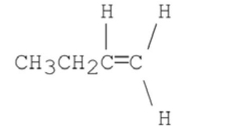
B)
C)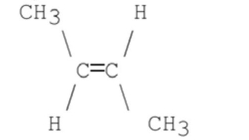
D)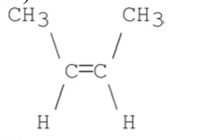
E)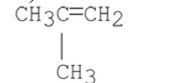
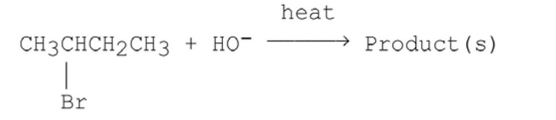
A)

B)
C)

D)

E)


Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
47
How many alkenes can be obtained as products (even in minor amounts) when the alkyl halide shown below undergoes an E2 reaction? (Count E/Z stereoisomers as different products.) 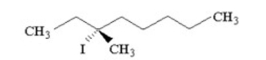
A) 1
B) 2
C) 3
D) 4
E) 5

A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
48
What is the major product of the following reaction?
A) 2,3-dimethyl-1-hexene
B) 2,3-dimethyl-2-hexene
C) 2 -isopropyl-1-pentene
D) (Z)-2,3 -dimethyl-3-hexene
E) (E)-2,3 -dimethyl-3-hexene

A) 2,3-dimethyl-1-hexene
B) 2,3-dimethyl-2-hexene
C) 2 -isopropyl-1-pentene
D) (Z)-2,3 -dimethyl-3-hexene
E) (E)-2,3 -dimethyl-3-hexene

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following alkyl bromides undergo SN1 olvolysis reactions?
A) (R)-2 -bromo-3-ethylpentane
B) (S)-2 -bromo-3-ethylpentane
C) (R)-3 -bromo-2-methylpentane
D) (S)-3 -bromo-2-methylpentane
E) 3-bromo-3-ethylpentane
A) (R)-2 -bromo-3-ethylpentane
B) (S)-2 -bromo-3-ethylpentane
C) (R)-3 -bromo-2-methylpentane
D) (S)-3 -bromo-2-methylpentane
E) 3-bromo-3-ethylpentane

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following are the elimination products obtained from the following reaction?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following alkyl halides reacts the fastest in an E2 reaction?
A)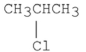
B)
C)
D)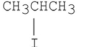
E)
A)

B)
C)
D)

E)

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following alkyl halides reacts the fastest in an E2 reaction?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
53
What kind of reaction is occurring below?
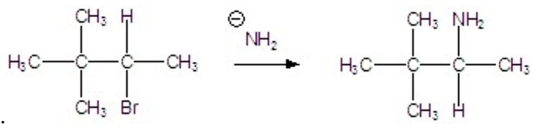
A) SN1
B) SN2
C) E1
D) E2
E) none of the above

A) SN1
B) SN2
C) E1
D) E2
E) none of the above

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
54
Using the following experimental data for the rate of the reaction given below:
![<strong>Using the following experimental data for the rate of the reaction given below: \begin{array} { | c | c | c | c | } \hline \text { Experiment \#1 } & \text { [Alkyl Halide] } & \text { [Base] } & \text { Rate } \\ \hline 1 & 0.01 & 0.01 & 1 \\ \hline 2 & 0.02 & 0.01 & 2 \\ \hline 3 & 0.01 & 0.02 & 1 \\ \hline \end{array} What kind of reaction is taking place?</strong> A) S<sub>N</sub>1 B) S<sub>N</sub>2 C) E1 D) E2 E) none of the above](https://d2lvgg3v3hfg70.cloudfront.net/TB1829/11ee043b_db62_f0cc_854b_2f8894cd27d5_TB1829_11.jpg)
What kind of reaction is taking place?
A) SN1
B) SN2
C) E1
D) E2
E) none of the above
![<strong>Using the following experimental data for the rate of the reaction given below: \begin{array} { | c | c | c | c | } \hline \text { Experiment \#1 } & \text { [Alkyl Halide] } & \text { [Base] } & \text { Rate } \\ \hline 1 & 0.01 & 0.01 & 1 \\ \hline 2 & 0.02 & 0.01 & 2 \\ \hline 3 & 0.01 & 0.02 & 1 \\ \hline \end{array} What kind of reaction is taking place?</strong> A) S<sub>N</sub>1 B) S<sub>N</sub>2 C) E1 D) E2 E) none of the above](https://d2lvgg3v3hfg70.cloudfront.net/TB1829/11ee043b_db62_f0cc_854b_2f8894cd27d5_TB1829_11.jpg)
What kind of reaction is taking place?
A) SN1
B) SN2
C) E1
D) E2
E) none of the above

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
55
What is the major product of the following reaction?
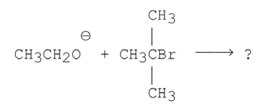
A)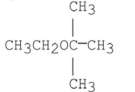
B)
C)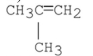
D) A and B
E) A and C

A)

B)
C)
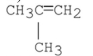
D) A and B
E) A and C

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following alkyl halides is the least reactive in an E2 reaction?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following is least likely to be formed as a product when 2-iodopentane reacts with sodium ethoxide?
A) 1-ethoxypentane
B) 2-ethoxypentane
C) (Z)-2-pentene
D) (E)-2-pentene
E) 1-pentene
A) 1-ethoxypentane
B) 2-ethoxypentane
C) (Z)-2-pentene
D) (E)-2-pentene
E) 1-pentene

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following alkyl halides undergoes SN1 solvolysis most rapidly?
A) cyclohexyl bromide
B) methyl iodide
C) isopropyl chloride
D) 3-chloropentane
E) 3-iodo-3-methylpentane
A) cyclohexyl bromide
B) methyl iodide
C) isopropyl chloride
D) 3-chloropentane
E) 3-iodo-3-methylpentane

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following best explains why SN1 reactions involving a neutral reactant are faster in polar solvents?
A) The reactant is more soluble in polar solvents.
B) The reactant is less soluble in polar solvents.
C) The nucleophile is solvated by polar solvents.
D) Solvation by polar solvents stabilizes the carbocation more than it stabilizes the transition state.
E) Solvation by polar solvents stabilizes the transition statemore than it stabilizes the neutral reactant.
A) The reactant is more soluble in polar solvents.
B) The reactant is less soluble in polar solvents.
C) The nucleophile is solvated by polar solvents.
D) Solvation by polar solvents stabilizes the carbocation more than it stabilizes the transition state.
E) Solvation by polar solvents stabilizes the transition statemore than it stabilizes the neutral reactant.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following alkyl iodides undergoes SN1 solvolysis most rapidly?
A) 1-iodo-3-methylpentane
B) 2-iodopentane
C) 2-iodo-2-methylpentane
D) 3 -iodopentane
E) 1 -iodo-2,2-dimethylpentane
A) 1-iodo-3-methylpentane
B) 2-iodopentane
C) 2-iodo-2-methylpentane
D) 3 -iodopentane
E) 1 -iodo-2,2-dimethylpentane

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
61
What is the substitution product of the following reaction? 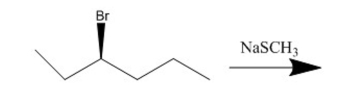


Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
62
Draw the product of the following SN2 eaction: 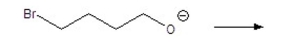


Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
63
Draw all possible alkene products of the following reaction and circle the major product: 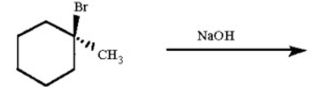


Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
64
What two reactions occur when 2-iodohexane reacts with sodium ethoxide?
A) SN2 and SN1
B)
C) SN2 and E2
D) E1 and SN1
E) E2 and E1
A) SN2 and SN1
B)
C) SN2 and E2
D) E1 and SN1
E) E2 and E1

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
65
What are the products of the following reaction?
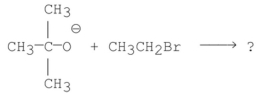
A)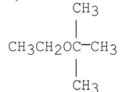
B)
C)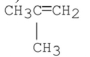
D) A and B
E) A and C
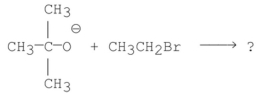
A)

B)
C)
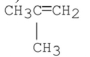
D) A and B
E) A and C

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
66
What are the products of the following reaction? Select all that apply.
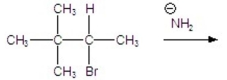
A)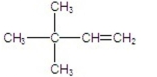
B)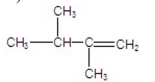
C)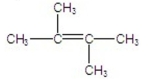
D)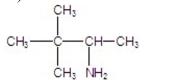
E)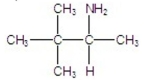
F)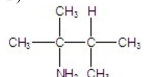

A)

B)

C)

D)

E)

F)


Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following statements is true about SN1 reactions of alkyl halides?
A) Complete inversion of configuration occurs.
B) The reactions are favored by nonpolar solvents.
C) The reactions are favored by polar solvents.
D) Reaction rates depend only on the concentration of the nucleophile.
E) The reaction occurs via a one-step back-side attack.
A) Complete inversion of configuration occurs.
B) The reactions are favored by nonpolar solvents.
C) The reactions are favored by polar solvents.
D) Reaction rates depend only on the concentration of the nucleophile.
E) The reaction occurs via a one-step back-side attack.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
68
Draw all possible alkene products of the following reaction and circle the major product: 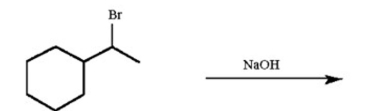


Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
69
Do all primary alkyl iodides underg SN2 eactions with sodium hydroxide in a given solvent at the same rate? Explain.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
70
Draw the substitution product of the following reaction: 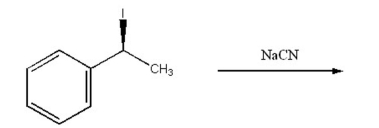


Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
71
Draw the substitution product of the following reaction: 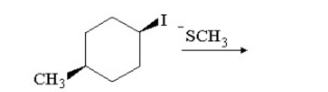


Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
72
Draw the substitution product of the following reaction: 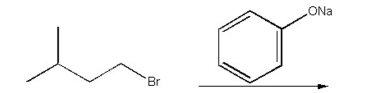


Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
73
Draw the substitution product of the following reaction: 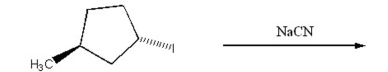
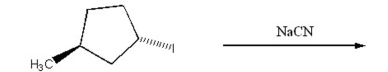

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
74
Draw the substitution product of the following reaction: 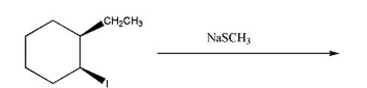


Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
75
For each of the following reactions, indicate the alkyl halide that will form the greatest amount of the given product: 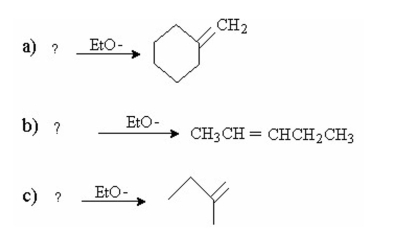


Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
76
What is the substitution product of the following reaction? 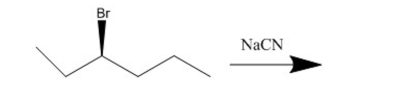


Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
77
Draw the substitution product of the following reaction: 


Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
78
Draw the substitution product of the following reaction: 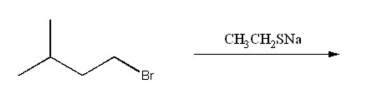


Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
79
When the nucleophile and the leaving group are contained in the same molecule, is an intermolecular or an intramolecular reaction more likely to occur? Explain.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
80
A student attempted to prepare 1-chlorobutane by reacting 1-butanol with a solution of NaCl. Did the student obtain the desired product? Explain.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck



