Deck 12: Reaction Rates and Chemical Equilibrium
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/52
Play
Full screen (f)
Deck 12: Reaction Rates and Chemical Equilibrium
1
Which of the following will not increase the rate of a reaction?
A)decreasing the temperature
B)decreasing the concentration of the reactants
C)decreasing the surface area of the reactants
D)removing a catalyst
E)All of these will not increase the rate of a reaction.
A)decreasing the temperature
B)decreasing the concentration of the reactants
C)decreasing the surface area of the reactants
D)removing a catalyst
E)All of these will not increase the rate of a reaction.
All of these will not increase the rate of a reaction.
2
The kinetics of a reaction is studied at 25 C and at 50 C.Which of the following statements is correct?
A)The rate of the reaction at 50 C will be lower than the rate at 25 C, since the molecules will be moving too fast to collide effectively.
B)The rate of the reaction at 50 C will be twice that of the rate at 25 C, since the temperature has doubled.
C)The rate of the reaction at 50 C will be greater than the rate at 25 C, since the activation energy will be lower at the higher temperature.
D)The rate of the reaction at 50 C will be greater than the rate at 25 C, since a greater fraction of the molecules will possess sufficient energy to react at the higher temperature.
E)None of these statements is correct.
A)The rate of the reaction at 50 C will be lower than the rate at 25 C, since the molecules will be moving too fast to collide effectively.
B)The rate of the reaction at 50 C will be twice that of the rate at 25 C, since the temperature has doubled.
C)The rate of the reaction at 50 C will be greater than the rate at 25 C, since the activation energy will be lower at the higher temperature.
D)The rate of the reaction at 50 C will be greater than the rate at 25 C, since a greater fraction of the molecules will possess sufficient energy to react at the higher temperature.
E)None of these statements is correct.
The rate of the reaction at 50 C will be greater than the rate at 25 C, since a greater fraction of the molecules will possess sufficient energy to react at the higher temperature.
3
In a chemical reaction at constant temperature, the addition of a catalyst
A)affects the equilibrium constant.
B)increases the average kinetic energy of the reactants.
C)provides an alternate reaction pathway with a different activation energy.
D)decreases the energy released in the chemical reaction.
E)increases the concentration of the products at equilibrium.
A)affects the equilibrium constant.
B)increases the average kinetic energy of the reactants.
C)provides an alternate reaction pathway with a different activation energy.
D)decreases the energy released in the chemical reaction.
E)increases the concentration of the products at equilibrium.
provides an alternate reaction pathway with a different activation energy.
4
Identify the intermediate in the following two-step reaction.Cr2+(aq) + Sn4+(aq) ⇌Cr3+(aq) + Sn3+(aq) Cr2+(aq) + Sn3+(aq) Cr3+(aq) + Sn2+(aq)
A)Sn3+(aq)
B)Sn4+(aq)
C)Sn2+(aq)
D)Cr2+(aq)
E)Cr3+(aq)
A)Sn3+(aq)
B)Sn4+(aq)
C)Sn2+(aq)
D)Cr2+(aq)
E)Cr3+(aq)

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
5
Identify any intermediates or catalysts in the following two-step reaction: S2O82- + I- 2SO42- + I+ I+ + I- I2
A)S2O82- is an intermediate.
B)S2O82- is a catalyst.
C)I- is a catalyst.
D)I+ is an intermediate.
E)SO42- is a catalyst.
A)S2O82- is an intermediate.
B)S2O82- is a catalyst.
C)I- is a catalyst.
D)I+ is an intermediate.
E)SO42- is a catalyst.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
6
Identify any intermediates in the following three-step reaction: H2C=CH2 + H3O+ H3C=CH2+ + H2O H3C=CH2+ + H2O CH3CH2OH2+ CH3CH2OH2+ + H2O CH3CH2OH + H3O+
A)H3O+ is an intermediate.
B)H3C=CH2+ is an intermediate.
C)H3C=CH2+, CH3CH2OH2+, and H2O are intermediates.
D)CH3CH2OH2+ is an intermediate.
E)H2O is an intermediate.
A)H3O+ is an intermediate.
B)H3C=CH2+ is an intermediate.
C)H3C=CH2+, CH3CH2OH2+, and H2O are intermediates.
D)CH3CH2OH2+ is an intermediate.
E)H2O is an intermediate.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
7
Identify the intermediate(s) in the following two-step reaction.O3(g) ⇌ O2(g) + O(g) O(g) + O3(g) 2O2(g)
A)O(g)
B)O3(g)
C)O2(g)
D)O2(g) and O(g)
E)O3(g) and O2(g)
A)O(g)
B)O3(g)
C)O2(g)
D)O2(g) and O(g)
E)O3(g) and O2(g)

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following changes will increase the fraction of collisions that are effective collisions?
A)increasing the temperature
B)increasing the concentration of the reactants
C)decreasing the concentration of the reactants
D)increasing the surface area of the reactants
E)All of these choices are correct.
A)increasing the temperature
B)increasing the concentration of the reactants
C)decreasing the concentration of the reactants
D)increasing the surface area of the reactants
E)All of these choices are correct.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
9
According to collision theory, the increase in the rate constant with increasing temperature is due mostly to the fact that
A)the activation energy decreases with increasing temperature.
B)the fraction of the collisions having sufficient energy to react increases with increasing temperature.
C)the pressure of the reactants increases with increasing temperature.
D)the heat change for most reactions is negative.
E)the fraction of the collisions that have the proper orientation for reaction increases with increasing temperature.
A)the activation energy decreases with increasing temperature.
B)the fraction of the collisions having sufficient energy to react increases with increasing temperature.
C)the pressure of the reactants increases with increasing temperature.
D)the heat change for most reactions is negative.
E)the fraction of the collisions that have the proper orientation for reaction increases with increasing temperature.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following statements regarding catalysts is incorrect?
A)A catalyst increases the rate of a reaction by giving the reaction an alternate pathway with a lower activation energy.
B)Catalysts need not be present in large amounts because they are regenerated during the reaction.
C)Enzymes act as catalysts in our bodies.
D)In a chemical reaction, the catalyst is shown on the reactant side of the equation.
E)The shape of an active site on an enzyme is unique, allowing it to react with only one substrate.
A)A catalyst increases the rate of a reaction by giving the reaction an alternate pathway with a lower activation energy.
B)Catalysts need not be present in large amounts because they are regenerated during the reaction.
C)Enzymes act as catalysts in our bodies.
D)In a chemical reaction, the catalyst is shown on the reactant side of the equation.
E)The shape of an active site on an enzyme is unique, allowing it to react with only one substrate.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
11
Identify any intermediates or catalysts in the following two-step reaction: H2O2 + 2Br- + 2H+ 2H2O + Br2 H2O2 + Br2 2H+ + O2 + 2Br-
A)H2O2 is an intermediate.
B)H2O2 is a catalyst, Br- is an intermediate.
C)Br- is a catalyst, Br2 is an intermediate.
D)H+ is a catalyst.
E)Br- and H+ are catalysts, Br2 is an intermediate.
A)H2O2 is an intermediate.
B)H2O2 is a catalyst, Br- is an intermediate.
C)Br- is a catalyst, Br2 is an intermediate.
D)H+ is a catalyst.
E)Br- and H+ are catalysts, Br2 is an intermediate.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
12
Identify the catalyst in the following three-step process for the decomposition of ozone.O3(g) ⇌ O2(g) + O(g) NO(g) + O3(g) NO2(g) + O2(g) O(g) + NO2(g) O2(g) + NO(g)
A)O(g)
B)O3(g)
C)O2(g)
D)NO(g)
E)NO2(g)
A)O(g)
B)O3(g)
C)O2(g)
D)NO(g)
E)NO2(g)

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
13
The rate of a reaction can be increased by all of the following except
A)increasing the temperature.
B)increasing the concentration of the reactants.
C)increasing the surface area of the reactants.
D)adding a catalyst.
E)increasing the volume of the reaction vessel.
A)increasing the temperature.
B)increasing the concentration of the reactants.
C)increasing the surface area of the reactants.
D)adding a catalyst.
E)increasing the volume of the reaction vessel.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following statements regarding catalysts is incorrect?
A)A catalyst increases the rate of a reaction by giving the reaction an alternate pathway with lower activation energy.
B)A catalyst is formed temporarily in an early step of a reaction.
C)Most enzyme catalysts are large protein molecules.
D)In a chemical equation the catalyst is shown above the reaction arrow of the equation.
E)The shape of an active site on an enzyme is unique, allowing it to react with only one substrate.
A)A catalyst increases the rate of a reaction by giving the reaction an alternate pathway with lower activation energy.
B)A catalyst is formed temporarily in an early step of a reaction.
C)Most enzyme catalysts are large protein molecules.
D)In a chemical equation the catalyst is shown above the reaction arrow of the equation.
E)The shape of an active site on an enzyme is unique, allowing it to react with only one substrate.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
15
Identify any intermediates or catalysts in the following two-step reaction: Cu2+ + H2 CuH+ + H+ CuH+ + H+ + H2C=CH2 Cu2+ + H3C-CH3
A)CuH+ is an intermediate.
B)Cu2+ is a catalyst, H+ is an intermediate.
C)Cu2+ is a catalyst, CuH+ is an intermediate.
D)Cu2+ is a catalyst, CuH+ and H+ are intermediates.
E)CuH+ and H+ are catalysts, Cu2+ is an intermediate.
A)CuH+ is an intermediate.
B)Cu2+ is a catalyst, H+ is an intermediate.
C)Cu2+ is a catalyst, CuH+ is an intermediate.
D)Cu2+ is a catalyst, CuH+ and H+ are intermediates.
E)CuH+ and H+ are catalysts, Cu2+ is an intermediate.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
16
Identify an intermediate in the following two-step reaction.Sn2+ + Fe3+ Sn3+ + Fe2+ Sn3+ + Fe3+ Sn4+ + Fe2+
A)Sn2+
B)Fe3+
C)Sn3+
D)Fe2+
E)Sn4+
A)Sn2+
B)Fe3+
C)Sn3+
D)Fe2+
E)Sn4+

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
17
Factors that influence reaction rates include all of the following except the
A)magnitude of the equilibrium constant.
B)reaction temperature.
C)presence of a catalyst.
D)size of solid reactant particles.
E)concentration of reactants.
A)magnitude of the equilibrium constant.
B)reaction temperature.
C)presence of a catalyst.
D)size of solid reactant particles.
E)concentration of reactants.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
18
Identify the intermediate(s) in the following three-step reaction.Cl2(g) ⇌ 2Cl(g) CHCl3(g) + Cl(g) HCl(g) + CCl3(g) CCl3(g) + Cl(g) CCl4(g)
A)CHCl3(g)
B)HCl(g)
C)Cl2(g)
D)Cl(g)
E)Cl(g) and CCl3(g)
A)CHCl3(g)
B)HCl(g)
C)Cl2(g)
D)Cl(g)
E)Cl(g) and CCl3(g)

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
19
A catalyst speeds up a chemical reaction by
A)providing an alternate reaction pathway.
B)increasing the activation energy.
C)shifting the equilibrium.
D)increasing the heat of the reaction.
E)decreasing the heat of the reaction.
A)providing an alternate reaction pathway.
B)increasing the activation energy.
C)shifting the equilibrium.
D)increasing the heat of the reaction.
E)decreasing the heat of the reaction.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
20
When a chemical system has reached equilibrium:
A)the forward and reverse reactions have stopped
B)the rate of the forward reaction is greater than the rate of the reverse reaction.
C)the rate of the forward reaction is less than the rate of the reverse reaction.
D)the rate of the forward reaction is equal to the rate of the reverse reaction.
E)the equilibrium constant has reached a minimum.
A)the forward and reverse reactions have stopped
B)the rate of the forward reaction is greater than the rate of the reverse reaction.
C)the rate of the forward reaction is less than the rate of the reverse reaction.
D)the rate of the forward reaction is equal to the rate of the reverse reaction.
E)the equilibrium constant has reached a minimum.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
21
Consider the reaction CO(g) + H2O(g) CO2(g) + H2(g), represented by the following diagram: 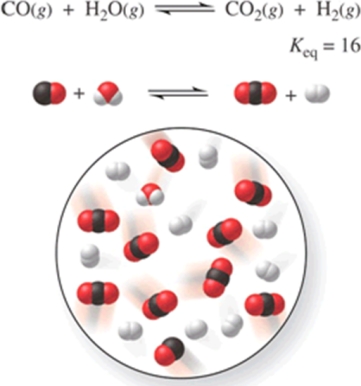 What is the composition of this system when the reaction reaches equilibrium?
What is the composition of this system when the reaction reaches equilibrium?
A)3 CO, 3 H2O, 7 CO2, 7 H2
B)1 CO, 1 H2O, 4 CO2, 4 H2
C)2 CO, 2 H2O, 8 CO2, 8 H2
D)8 CO, 8 H2O, 2 CO2, 2 H2
E)4 CO, 6 H2O, 4 CO2, 6 H2
 What is the composition of this system when the reaction reaches equilibrium?
What is the composition of this system when the reaction reaches equilibrium?A)3 CO, 3 H2O, 7 CO2, 7 H2
B)1 CO, 1 H2O, 4 CO2, 4 H2
C)2 CO, 2 H2O, 8 CO2, 8 H2
D)8 CO, 8 H2O, 2 CO2, 2 H2
E)4 CO, 6 H2O, 4 CO2, 6 H2

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following statements is correct for a reaction in which Keq << 1?
A)The forward reaction is faster than the reverse reaction.
B)The reverse reaction is faster than the forward reaction.
C)There are more products than reactants at equilibrium.
D)There are more reactants than products at equilibrium.
E)None of these statements is correct.
A)The forward reaction is faster than the reverse reaction.
B)The reverse reaction is faster than the forward reaction.
C)There are more products than reactants at equilibrium.
D)There are more reactants than products at equilibrium.
E)None of these statements is correct.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
23
Given that evaporation is an endothermic process, which of the following sets of changes would cause the following system at equilibrium to shift to increase the number of I2 molecules in the gas phase? I2(s) ⇌ I2(g)
A)Increasing container volume and increasing temperature
B)Increasing container volume and decreasing temperature
C)Decreasing container volume and increasing temperature
D)Decreasing container volume and decreasing temperature
E)Changing volume or temperature will not affect the number of gas molecules present at equilibrium
A)Increasing container volume and increasing temperature
B)Increasing container volume and decreasing temperature
C)Decreasing container volume and increasing temperature
D)Decreasing container volume and decreasing temperature
E)Changing volume or temperature will not affect the number of gas molecules present at equilibrium

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
24
Which equilibrium constant represents a reaction that is reactant favored?
A)Keq = 0.025
B)Keq = 5.2
C)Keq = 8.4 x 10-5
D)Keq = 6.3 x 105
E)not enough information
A)Keq = 0.025
B)Keq = 5.2
C)Keq = 8.4 x 10-5
D)Keq = 6.3 x 105
E)not enough information

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
25
The graph shows the change in concentrations of the reactant and product as a reaction proceeds.At what point is equilibrium first reached? 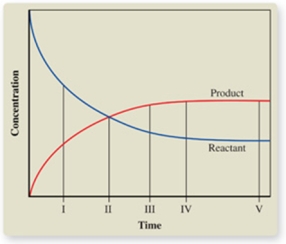
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
26
The amount of energy that reactant molecules must possess in order to react is the activation energy.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
27
Consider the following reaction carried out in a sealed container: N2(g) + 3H2(g) ⇌ 2NH3(g) A state of equilibrium can be reached if the container initially contains
A)NH3 only.
B)N2 and H2 only.
C)NH3 and N2 only.
D)NH3, N2, and H2.
E)any of these combinations of reactants and product.
A)NH3 only.
B)N2 and H2 only.
C)NH3 and N2 only.
D)NH3, N2, and H2.
E)any of these combinations of reactants and product.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
28
A reaction that has a high activation energy will have a high rate of reaction.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following statements is correct for a reaction in which Keq >> 1?
A)The forward reaction is faster than the reverse reaction.
B)The reverse reaction is faster than the forward reaction.
C)There are more products than reactants at equilibrium.
D)There are more reactants than products at equilibrium.
E)None of these statements is correct.
A)The forward reaction is faster than the reverse reaction.
B)The reverse reaction is faster than the forward reaction.
C)There are more products than reactants at equilibrium.
D)There are more reactants than products at equilibrium.
E)None of these statements is correct.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
30
Which equilibrium constant represents a reaction that is product favored?
A)Keq = 0.025
B)Keq = 5.2
C)Keq = 8.4 x 10-5
D)Keq = 6.3 x 105
E)not enough information
A)Keq = 0.025
B)Keq = 5.2
C)Keq = 8.4 x 10-5
D)Keq = 6.3 x 105
E)not enough information

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
31
Given that the following reaction is exothermic, which of the following sets of changes would cause the following system at equilibrium to shift to increase the number of N2O4(g) molecules? 2NO2(g) ⇌N2O4(g) What observation would indicate that the reaction is at equilibrium in a closed container?
A)Increasing container volume and increasing temperature
B)Increasing container volume and decreasing temperature
C)Decreasing container volume and increasing temperature
D)Decreasing container volume and decreasing temperature
E)Changing volume or temperature will not affect the number of gas molecules present at equilibrium
A)Increasing container volume and increasing temperature
B)Increasing container volume and decreasing temperature
C)Decreasing container volume and increasing temperature
D)Decreasing container volume and decreasing temperature
E)Changing volume or temperature will not affect the number of gas molecules present at equilibrium

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
32
Consider the following reaction carried out in a sealed container: N2(g) + 3H2(g) ⇌ 2NH3(g) A state of equilibrium cannot be reached if the container initially contains
A)NH3 only.
B)N2 only.
C)NH3 and N2 only.
D)NH3, N2, and H2.
A)NH3 only.
B)N2 only.
C)NH3 and N2 only.
D)NH3, N2, and H2.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
33
Select the correct equilibrium constant expression for the following reaction: H2(g) + I2(g) ⇌ 2HI(g)
A)![<strong>Select the correct equilibrium constant expression for the following reaction: H<sub>2</sub>(g) + I<sub>2</sub>(g) ⇌ 2HI(g)</strong> A) B)K<sub>eq</sub> = [HI]<sup>2</sup> [H<sub>2</sub>][I<sub>2</sub>] C)K<sub>eq</sub> = [HI]<sup>2</sup> + [H<sub>2</sub>] + [I<sub>2</sub>] D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB7010/11eb4adb_00b4_e42d_99b2_7d2b7060bcd5_TB7010_11.jpg)
B)Keq = [HI]2 [H2][I2]
C)Keq = [HI]2 + [H2] + [I2]
D)![<strong>Select the correct equilibrium constant expression for the following reaction: H<sub>2</sub>(g) + I<sub>2</sub>(g) ⇌ 2HI(g)</strong> A) B)K<sub>eq</sub> = [HI]<sup>2</sup> [H<sub>2</sub>][I<sub>2</sub>] C)K<sub>eq</sub> = [HI]<sup>2</sup> + [H<sub>2</sub>] + [I<sub>2</sub>] D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB7010/11eb4adb_00b4_e42e_99b2_b53b527aeb44_TB7010_11.jpg)
E)![<strong>Select the correct equilibrium constant expression for the following reaction: H<sub>2</sub>(g) + I<sub>2</sub>(g) ⇌ 2HI(g)</strong> A) B)K<sub>eq</sub> = [HI]<sup>2</sup> [H<sub>2</sub>][I<sub>2</sub>] C)K<sub>eq</sub> = [HI]<sup>2</sup> + [H<sub>2</sub>] + [I<sub>2</sub>] D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB7010/11eb4adb_00b4_e42f_99b2_0f7e195777f1_TB7010_11.jpg)
A)
![<strong>Select the correct equilibrium constant expression for the following reaction: H<sub>2</sub>(g) + I<sub>2</sub>(g) ⇌ 2HI(g)</strong> A) B)K<sub>eq</sub> = [HI]<sup>2</sup> [H<sub>2</sub>][I<sub>2</sub>] C)K<sub>eq</sub> = [HI]<sup>2</sup> + [H<sub>2</sub>] + [I<sub>2</sub>] D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB7010/11eb4adb_00b4_e42d_99b2_7d2b7060bcd5_TB7010_11.jpg)
B)Keq = [HI]2 [H2][I2]
C)Keq = [HI]2 + [H2] + [I2]
D)
![<strong>Select the correct equilibrium constant expression for the following reaction: H<sub>2</sub>(g) + I<sub>2</sub>(g) ⇌ 2HI(g)</strong> A) B)K<sub>eq</sub> = [HI]<sup>2</sup> [H<sub>2</sub>][I<sub>2</sub>] C)K<sub>eq</sub> = [HI]<sup>2</sup> + [H<sub>2</sub>] + [I<sub>2</sub>] D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB7010/11eb4adb_00b4_e42e_99b2_b53b527aeb44_TB7010_11.jpg)
E)
![<strong>Select the correct equilibrium constant expression for the following reaction: H<sub>2</sub>(g) + I<sub>2</sub>(g) ⇌ 2HI(g)</strong> A) B)K<sub>eq</sub> = [HI]<sup>2</sup> [H<sub>2</sub>][I<sub>2</sub>] C)K<sub>eq</sub> = [HI]<sup>2</sup> + [H<sub>2</sub>] + [I<sub>2</sub>] D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB7010/11eb4adb_00b4_e42f_99b2_0f7e195777f1_TB7010_11.jpg)

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
34
Select the correct equilibrium constant expression for the reaction 4NH3(g) + 3O2(g) ⇌ 2N2(g) + 6H2O(g)
A)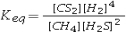
B)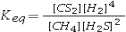
C)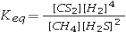
D)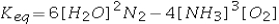
E)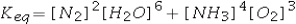
A)
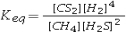
B)
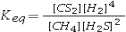
C)
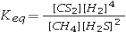
D)
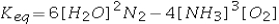
E)
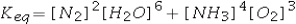

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
35
In order to write the equilibrium constant expression for a reaction, you must:
A)know the mechanism for the reaction.
B)know the rate of the forward and reverse reactions.
C)know the concentrations of all reactants and products.
D)have the balanced chemical equation for the reaction.
E)know the conditions of pressure, temperature, and concentration for the system.
A)know the mechanism for the reaction.
B)know the rate of the forward and reverse reactions.
C)know the concentrations of all reactants and products.
D)have the balanced chemical equation for the reaction.
E)know the conditions of pressure, temperature, and concentration for the system.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
36
Select the correct equilibrium constant expression for the reaction: CH4(g) + 2H2S(g) ⇌ CS2(g) + 4H2(g)
A)![<strong>Select the correct equilibrium constant expression for the reaction: CH<sub>4</sub>(g) + 2H<sub>2</sub>S(g) ⇌ CS<sub>2</sub>(g) + 4H<sub>2</sub>(g)</strong> A) B) C) D)K<sub>eq</sub> = [CS<sub>2</sub>][H<sub>2</sub>]<sup>4</sup> - [CH<sub>4</sub>][H<sub>2</sub>S]<sup>2</sup> E)K<sub>eq</sub> = [CS<sub>2</sub>][H<sub>2</sub>]<sup>4</sup> + [CH<sub>4</sub>][H<sub>2</sub>S]<sup>2</sup><sup> </sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB7010/11eb4adb_00b5_0b40_99b2_d182e5540a46_TB7010_11.jpg)
![<strong>Select the correct equilibrium constant expression for the reaction: CH<sub>4</sub>(g) + 2H<sub>2</sub>S(g) ⇌ CS<sub>2</sub>(g) + 4H<sub>2</sub>(g)</strong> A) B) C) D)K<sub>eq</sub> = [CS<sub>2</sub>][H<sub>2</sub>]<sup>4</sup> - [CH<sub>4</sub>][H<sub>2</sub>S]<sup>2</sup> E)K<sub>eq</sub> = [CS<sub>2</sub>][H<sub>2</sub>]<sup>4</sup> + [CH<sub>4</sub>][H<sub>2</sub>S]<sup>2</sup><sup> </sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB7010/11eb4adb_00b5_0b41_99b2_535b03824566_TB7010_11.jpg)
B)![<strong>Select the correct equilibrium constant expression for the reaction: CH<sub>4</sub>(g) + 2H<sub>2</sub>S(g) ⇌ CS<sub>2</sub>(g) + 4H<sub>2</sub>(g)</strong> A) B) C) D)K<sub>eq</sub> = [CS<sub>2</sub>][H<sub>2</sub>]<sup>4</sup> - [CH<sub>4</sub>][H<sub>2</sub>S]<sup>2</sup> E)K<sub>eq</sub> = [CS<sub>2</sub>][H<sub>2</sub>]<sup>4</sup> + [CH<sub>4</sub>][H<sub>2</sub>S]<sup>2</sup><sup> </sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB7010/11eb4adb_00b5_0b42_99b2_c37d79f59539_TB7010_11.jpg)
![<strong>Select the correct equilibrium constant expression for the reaction: CH<sub>4</sub>(g) + 2H<sub>2</sub>S(g) ⇌ CS<sub>2</sub>(g) + 4H<sub>2</sub>(g)</strong> A) B) C) D)K<sub>eq</sub> = [CS<sub>2</sub>][H<sub>2</sub>]<sup>4</sup> - [CH<sub>4</sub>][H<sub>2</sub>S]<sup>2</sup> E)K<sub>eq</sub> = [CS<sub>2</sub>][H<sub>2</sub>]<sup>4</sup> + [CH<sub>4</sub>][H<sub>2</sub>S]<sup>2</sup><sup> </sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB7010/11eb4adb_00b5_0b43_99b2_61d2544acf04_TB7010_11.jpg)
C)![<strong>Select the correct equilibrium constant expression for the reaction: CH<sub>4</sub>(g) + 2H<sub>2</sub>S(g) ⇌ CS<sub>2</sub>(g) + 4H<sub>2</sub>(g)</strong> A) B) C) D)K<sub>eq</sub> = [CS<sub>2</sub>][H<sub>2</sub>]<sup>4</sup> - [CH<sub>4</sub>][H<sub>2</sub>S]<sup>2</sup> E)K<sub>eq</sub> = [CS<sub>2</sub>][H<sub>2</sub>]<sup>4</sup> + [CH<sub>4</sub>][H<sub>2</sub>S]<sup>2</sup><sup> </sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB7010/11eb4adb_00b5_0b44_99b2_0157d92743b7_TB7010_11.jpg)
D)Keq = [CS2][H2]4 - [CH4][H2S]2
E)Keq = [CS2][H2]4 + [CH4][H2S]2
A)
![<strong>Select the correct equilibrium constant expression for the reaction: CH<sub>4</sub>(g) + 2H<sub>2</sub>S(g) ⇌ CS<sub>2</sub>(g) + 4H<sub>2</sub>(g)</strong> A) B) C) D)K<sub>eq</sub> = [CS<sub>2</sub>][H<sub>2</sub>]<sup>4</sup> - [CH<sub>4</sub>][H<sub>2</sub>S]<sup>2</sup> E)K<sub>eq</sub> = [CS<sub>2</sub>][H<sub>2</sub>]<sup>4</sup> + [CH<sub>4</sub>][H<sub>2</sub>S]<sup>2</sup><sup> </sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB7010/11eb4adb_00b5_0b40_99b2_d182e5540a46_TB7010_11.jpg)
![<strong>Select the correct equilibrium constant expression for the reaction: CH<sub>4</sub>(g) + 2H<sub>2</sub>S(g) ⇌ CS<sub>2</sub>(g) + 4H<sub>2</sub>(g)</strong> A) B) C) D)K<sub>eq</sub> = [CS<sub>2</sub>][H<sub>2</sub>]<sup>4</sup> - [CH<sub>4</sub>][H<sub>2</sub>S]<sup>2</sup> E)K<sub>eq</sub> = [CS<sub>2</sub>][H<sub>2</sub>]<sup>4</sup> + [CH<sub>4</sub>][H<sub>2</sub>S]<sup>2</sup><sup> </sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB7010/11eb4adb_00b5_0b41_99b2_535b03824566_TB7010_11.jpg)
B)
![<strong>Select the correct equilibrium constant expression for the reaction: CH<sub>4</sub>(g) + 2H<sub>2</sub>S(g) ⇌ CS<sub>2</sub>(g) + 4H<sub>2</sub>(g)</strong> A) B) C) D)K<sub>eq</sub> = [CS<sub>2</sub>][H<sub>2</sub>]<sup>4</sup> - [CH<sub>4</sub>][H<sub>2</sub>S]<sup>2</sup> E)K<sub>eq</sub> = [CS<sub>2</sub>][H<sub>2</sub>]<sup>4</sup> + [CH<sub>4</sub>][H<sub>2</sub>S]<sup>2</sup><sup> </sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB7010/11eb4adb_00b5_0b42_99b2_c37d79f59539_TB7010_11.jpg)
![<strong>Select the correct equilibrium constant expression for the reaction: CH<sub>4</sub>(g) + 2H<sub>2</sub>S(g) ⇌ CS<sub>2</sub>(g) + 4H<sub>2</sub>(g)</strong> A) B) C) D)K<sub>eq</sub> = [CS<sub>2</sub>][H<sub>2</sub>]<sup>4</sup> - [CH<sub>4</sub>][H<sub>2</sub>S]<sup>2</sup> E)K<sub>eq</sub> = [CS<sub>2</sub>][H<sub>2</sub>]<sup>4</sup> + [CH<sub>4</sub>][H<sub>2</sub>S]<sup>2</sup><sup> </sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB7010/11eb4adb_00b5_0b43_99b2_61d2544acf04_TB7010_11.jpg)
C)
![<strong>Select the correct equilibrium constant expression for the reaction: CH<sub>4</sub>(g) + 2H<sub>2</sub>S(g) ⇌ CS<sub>2</sub>(g) + 4H<sub>2</sub>(g)</strong> A) B) C) D)K<sub>eq</sub> = [CS<sub>2</sub>][H<sub>2</sub>]<sup>4</sup> - [CH<sub>4</sub>][H<sub>2</sub>S]<sup>2</sup> E)K<sub>eq</sub> = [CS<sub>2</sub>][H<sub>2</sub>]<sup>4</sup> + [CH<sub>4</sub>][H<sub>2</sub>S]<sup>2</sup><sup> </sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB7010/11eb4adb_00b5_0b44_99b2_0157d92743b7_TB7010_11.jpg)
D)Keq = [CS2][H2]4 - [CH4][H2S]2
E)Keq = [CS2][H2]4 + [CH4][H2S]2

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
37
For a given reaction, which of the following statements can be made about the value of the equilibrium constant?
A)It always remains the same.
B)It increases when the concentration of one of the products is increased.
C)It increases when the concentration of one of the reactants is increased.
D)It changes when temperature is changed.
E)It can be changed by addition of a catalyst.
A)It always remains the same.
B)It increases when the concentration of one of the products is increased.
C)It increases when the concentration of one of the reactants is increased.
D)It changes when temperature is changed.
E)It can be changed by addition of a catalyst.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
38
Consider the following reaction carried out in a sealed container: 2SO3(g) ⇌ 2SO2(g) + O2(g) A state of equilibrium can be reached if the container initially contains
A)SO3 only
B)SO2 and O2 only.
C)SO3 and O2 only.
D)SO3, SO2, and O2.
E)any of these combinations of reactants and products.
A)SO3 only
B)SO2 and O2 only.
C)SO3 and O2 only.
D)SO3, SO2, and O2.
E)any of these combinations of reactants and products.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
39
The high potential-energy chemical species that is produced when an effective collision occurs is called a catalyst.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
40
Consider the following reaction carried out in a sealed container: 2SO3(g) ⇌ 2SO2(g) + O2(g) A state of equilibrium cannot be reached if the container initially contains
A)SO3 only.
B)O2 only.
C)SO2 and O2 only.
D)SO3 and O2 only.
E)SO3 , SO2 and O2.
A)SO3 only.
B)O2 only.
C)SO2 and O2 only.
D)SO3 and O2 only.
E)SO3 , SO2 and O2.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
41
Catalysts are not included in an overall chemical reaction because they are regenerated during the reaction.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
42
When we say that the position of equilibrium lies to the left, it means that there are fewer reactants than products at equilibrium.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
43
A catalyst gives an alternate pathway for a reaction that has a lower activation energy.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
44
The reaction CaCO3(s)  CaO(s) + CO2(g) is an example of a heterogeneous equilibrium.
CaO(s) + CO2(g) is an example of a heterogeneous equilibrium.
 CaO(s) + CO2(g) is an example of a heterogeneous equilibrium.
CaO(s) + CO2(g) is an example of a heterogeneous equilibrium.
Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
45
If heat is added to the system of an endothermic reaction, the equilibrium will shift to make more reactants.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
46
If the average energy of the products of a chemical reaction is greater than the average energy of the reactants, then the reaction is exothermic.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
47
The enzyme sucrase catalyzes the metabolism of sucrose.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
48
The only condition which can cause the equilibrium constant for a specific reaction to change is a temperature change.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
49
An activated complex is a short-lived, high-energy species that is formed during the process of a chemical reaction.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
50
A catalyst increases the rate of a reaction by increasing the kinetic energy of the reactants.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
51
An intermediate in a chemical reaction is a species that the reactant molecules interact with during an early step in a reaction that is regenerated during a later step in the reaction.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
52
When square brackets are placed around the formula for a substance in an equilibrium constant expression, it indicates the percent-by-mass concentration of that substance.For example [NaOH] means the percent-by-mass concentration of NaOH.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck



