Deck 8: Chemical Bonding
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/114
Play
Full screen (f)
Deck 8: Chemical Bonding
1
Which of the following compounds is likely to have covalent bonds?
A)NaCl
B)LiF
C)CO
D)CaBr2
E)MgO
A)NaCl
B)LiF
C)CO
D)CaBr2
E)MgO
CO
2
Which image(s) in the figure represents a compound with covalent bonds? 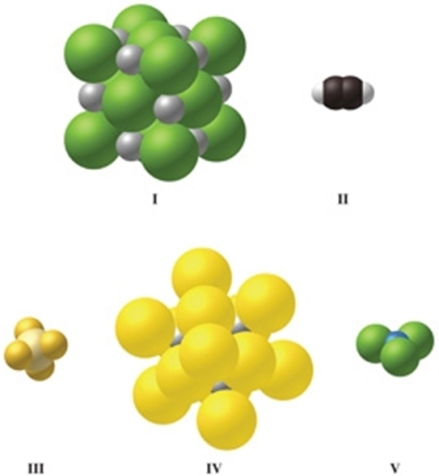
A)II only
B)III only
C)IV only
D)II and III
E)II, III, and V

A)II only
B)III only
C)IV only
D)II and III
E)II, III, and V
II, III, and V
3
Which image(s) in the figure represents an ionic compound? 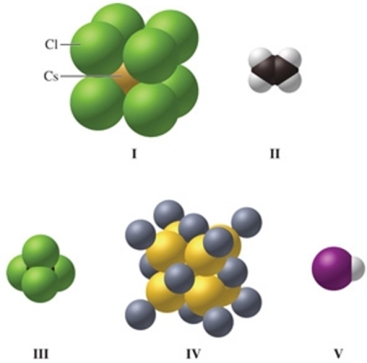
A)I only
B)II only
C)III only
D)IV only
E)I and IV

A)I only
B)II only
C)III only
D)IV only
E)I and IV
I and IV
4
In which of the following bonds does hydrogen have a partial negative charge ( -)?
A)H-F
B)H-B
C)H-C
D)H-H
E)None of these
A)H-F
B)H-B
C)H-C
D)H-H
E)None of these

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following substances has both ionic and covalent bonding?
A)NaCl
B)Cl2
C)MgO
D)MgCO3
E)None of these
A)NaCl
B)Cl2
C)MgO
D)MgCO3
E)None of these

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following compounds is likely to occur as a gas at room temperature?
A)CaF2
B)MgS
C)NO2
D)NaF
E)CoCl2
A)CaF2
B)MgS
C)NO2
D)NaF
E)CoCl2

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
7
In which of the following bonds does nitrogen have a partial negative charge ( -)?
A)N-C
B)N-O
C)N-F
D)N-N
E)None of these
A)N-C
B)N-O
C)N-F
D)N-N
E)None of these

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following compounds is likely to occur as a solid at room temperature?
A)CH4
B)SO2
C)CaCl2
D)CO2
E)H2O2
A)CH4
B)SO2
C)CaCl2
D)CO2
E)H2O2

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following compounds is likely to have a relatively high boiling point?
A)PH3
B)FeCl3
C)NO
D)SO2
E)C2H6
A)PH3
B)FeCl3
C)NO
D)SO2
E)C2H6

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following compounds is likely to have covalent bonds?
A)MgS
B)KF
C)SO2
D)SrCl2
E)RbF
A)MgS
B)KF
C)SO2
D)SrCl2
E)RbF

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
11
Which image(s) in the figure represents an ionic compound? 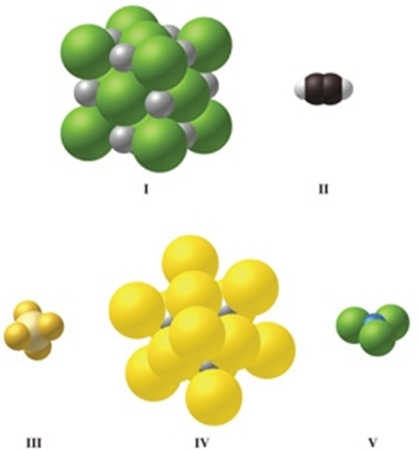
A)I only
B)II only
C)III only
D)IV only
E)I and IV

A)I only
B)II only
C)III only
D)IV only
E)I and IV

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following substances has bonding best described as ionic bonding?
A)SiO2
B)TiO2
C)SO2
D)CO2
E)O2
A)SiO2
B)TiO2
C)SO2
D)CO2
E)O2

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
13
Which image(s) in the figure represents a compound that contains covalent bonds? 
A)I only
B)II only
C)II and III
D)II, III, and V
E)V only

A)I only
B)II only
C)II and III
D)II, III, and V
E)V only

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following compounds is likely to occur as a solid at room temperature?
A)PH3
B)NO2
C)MgCl2
D)SO2
E)ClO2
A)PH3
B)NO2
C)MgCl2
D)SO2
E)ClO2

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following compounds is likely to have covalent bonds?
A)LiBr
B)CsF
C)BaCl2
D)Cr2O3
E)NO2
A)LiBr
B)CsF
C)BaCl2
D)Cr2O3
E)NO2

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following compounds is likely to have covalent bonds?
A)CaBr2
B)OF2
C)NaF
D)BaBr2
E)LiCl
A)CaBr2
B)OF2
C)NaF
D)BaBr2
E)LiCl

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following elements is the most electronegative?
A)carbon
B)silicon
C)nitrogen
D)oxygen
E)lithium
A)carbon
B)silicon
C)nitrogen
D)oxygen
E)lithium

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following elements is the most electronegative?
A)H
B)S
C)F
D)Cl
E)P
A)H
B)S
C)F
D)Cl
E)P

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following compounds is likely to occur as a gas at room temperature?
A)CaF2
B)CH4
C)MgO
D)FeCl3
E)LiF
A)CaF2
B)CH4
C)MgO
D)FeCl3
E)LiF

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following compounds is likely to have a relatively high boiling point?
A)CoCl3
B)NH3
C)CO
D)NO2
E)CH4
A)CoCl3
B)NH3
C)CO
D)NO2
E)CH4

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
21
The Lewis symbol for P3- is: 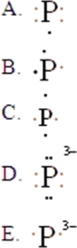
A)A
B)B
C)C
D)D
E)E
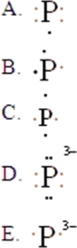
A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
22
Using periodic trends, arrange the following atoms in order of increasing electronegativity: S, Sr, P, Cs
A)S < Sr < P < Cs
B)S < P < Sr < Cs
C)P < Cs < Sr < S
D)Cs < Sr < S < P
E)Cs < Sr < P < S
A)S < Sr < P < Cs
B)S < P < Sr < Cs
C)P < Cs < Sr < S
D)Cs < Sr < S < P
E)Cs < Sr < P < S

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
23
In which of the following molecules does oxygen have a partial positive charge ( +)?
A)O2
B)OF2
C)H2O
D)MgO
E)None of these
A)O2
B)OF2
C)H2O
D)MgO
E)None of these

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
24
Using periodic trends, arrange the following atoms in order of increasing electronegativity: Cl, Si, Ga, Sr
A)Cl < Si < Ga < Sr
B)Si < Ga < Sr < Cl
C)Si < Cl < Ga < Sr
D)Sr < Ga < Si < Cl
E)Sr > Ga < Cl < Si
A)Cl < Si < Ga < Sr
B)Si < Ga < Sr < Cl
C)Si < Cl < Ga < Sr
D)Sr < Ga < Si < Cl
E)Sr > Ga < Cl < Si

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
25
 Arrange the following bonds in order of increasing polarity: O-H, C-H, F-H, H-H
Arrange the following bonds in order of increasing polarity: O-H, C-H, F-H, H-HA)O-H < C-H < F-H < H-H
B)C-H < O-H < F-H < H-H
C)H-H < C-H < O-H < F-H
D)C-H < H-H < O-H < F-H
E)H-H < C-H < F-H < O-H

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
26
The Lewis symbol for N is: 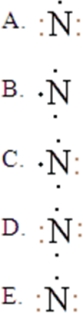
A)A
B)B
C)C
D)D
E)E
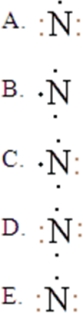
A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
27
The Lewis symbol for C is: 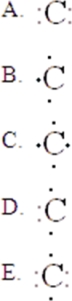
A)A
B)B
C)C
D)D
E)E
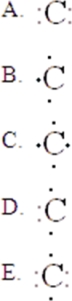
A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
28
Arrange the following bonds in order of increasing polarity: Cl-S, Cl-P, Cl-Si, Cl-Cl
A)Cl-S < Cl-P < Cl-Si < Cl-Cl
B)Cl-S < Cl-Si < Cl-P < Cl-Cl
C)Cl-Si < Cl-S < Cl-Cl < Cl-P
D)Cl-Cl < Cl-S < Cl-P < Cl-Si
E)Cl-Cl < Cl-P < Cl-Si < Cl-S
A)Cl-S < Cl-P < Cl-Si < Cl-Cl
B)Cl-S < Cl-Si < Cl-P < Cl-Cl
C)Cl-Si < Cl-S < Cl-Cl < Cl-P
D)Cl-Cl < Cl-S < Cl-P < Cl-Si
E)Cl-Cl < Cl-P < Cl-Si < Cl-S

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
29
Arrange the following bonds in order of increasing polarity: F-F, F-C, F-O, F-N
A)F-F < F-C < F-O < F-N
B)F-F < F-O < F-N < F-C
C)F-O < F-N < F-F < F-C
D)F-N < F-O < F-F < F-C
E)F-N < F-C < F-O < F-F
A)F-F < F-C < F-O < F-N
B)F-F < F-O < F-N < F-C
C)F-O < F-N < F-F < F-C
D)F-N < F-O < F-F < F-C
E)F-N < F-C < F-O < F-F

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
30
Arrange the following bonds in order of increasing polarity: C-O, C-C, C-N, C-F
A)C-O < C-C < C-N < C-F
B)C-N < C-O < C-F < C-C
C)C-F < C-O < C-N < C-C
D)C-C < C-N < C-O < C-F
E)C-C < C-O < C-N < C-F
A)C-O < C-C < C-N < C-F
B)C-N < C-O < C-F < C-C
C)C-F < C-O < C-N < C-C
D)C-C < C-N < C-O < C-F
E)C-C < C-O < C-N < C-F

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
31
The Lewis symbol for Al3+ is: 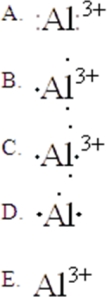
A)A
B)B
C)C
D)D
E)E
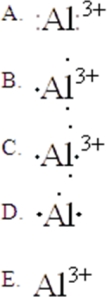
A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
32
Arrange the following bonds in order of increasing polarity: H-Cl, H-I, H-Br, H-F
A)H-Cl < H-I < H-Br < H-F
B)H-Cl < H-Br < H-I < H-F
C)H-F < H-Cl < H-Br < H-I
D)H-I < H-Br < H-Cl < H-F
E)H-I < H-Cl < H-Br < H-F
A)H-Cl < H-I < H-Br < H-F
B)H-Cl < H-Br < H-I < H-F
C)H-F < H-Cl < H-Br < H-I
D)H-I < H-Br < H-Cl < H-F
E)H-I < H-Cl < H-Br < H-F

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
33
The Lewis symbol for O2- is: 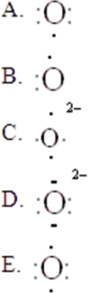
A)A
B)B
C)C
D)D
E)E
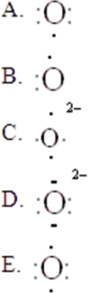
A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
34
The Lewis symbol for S is: 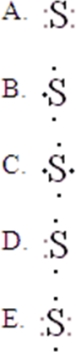
A)A
B)B
C)C
D)D
E)E
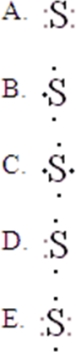
A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following molecules contains the most polar bonds?
A)F2
B)OF2
C)NF3
D)BF3
E)CF4
A)F2
B)OF2
C)NF3
D)BF3
E)CF4

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
36
Using periodic trends, arrange the following atoms in order of increasing electronegativity: F, O, S, As
A)F < O < S < As
B)As < O < S < F
C)As < S < O < F
D)S < As < O < F
E)S < As < F < O
A)F < O < S < As
B)As < O < S < F
C)As < S < O < F
D)S < As < O < F
E)S < As < F < O

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
37
The Lewis symbol for F is: 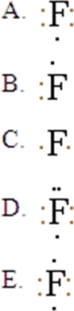
A)A
B)B
C)C
D)D
E)E
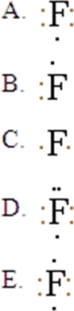
A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
38
The Lewis symbol for Mg is: 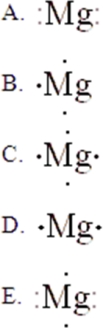
A)A
B)B
C)C
D)D
E)E
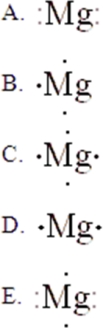
A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
39
The Lewis symbol for Ba2+ is: 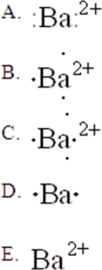
A)A
B)B
C)C
D)D
E)E
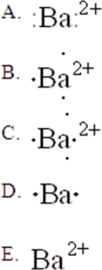
A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
40
Using periodic trends, arrange the following atoms in order of increasing electronegativity: Se, Cl, Mg, Na
A)Se < Cl < Mg < Na
B)Na < Mg < Cl < Se
C)Mg < Na < Se < Cl
D)Se < Mg < Na < Cl
E)Na < Mg < Se < Cl
A)Se < Cl < Mg < Na
B)Na < Mg < Cl < Se
C)Mg < Na < Se < Cl
D)Se < Mg < Na < Cl
E)Na < Mg < Se < Cl

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
41
How many single bonds are typically formed by the element C?
A)1
B)2
C)3
D)4
E)the number of bonds varies
A)1
B)2
C)3
D)4
E)the number of bonds varies

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following statements regarding covalent bonding is incorrect?
A)All atoms in a Lewis structure will have eight electrons around their element symbol.
B)Covalent bonds occur between two nonmetals.
C)Two atoms may share up to three pairs of electrons.
D)Carbon typically forms four bonds.
E)The elements which exist as diatomic molecules do so in order to achieve a stable electron configuration.
A)All atoms in a Lewis structure will have eight electrons around their element symbol.
B)Covalent bonds occur between two nonmetals.
C)Two atoms may share up to three pairs of electrons.
D)Carbon typically forms four bonds.
E)The elements which exist as diatomic molecules do so in order to achieve a stable electron configuration.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
43
Identify the main-group element X that could form the compound 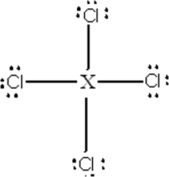
A)C
B)O
C)N
D)F
E)P

A)C
B)O
C)N
D)F
E)P

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
44
How many single bonds are typically formed by the element N?
A)1
B)2
C)3
D)4
E)the number of bonds varies
A)1
B)2
C)3
D)4
E)the number of bonds varies

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
45
How many single bonds are typically formed by the element O?
A)1
B)2
C)3
D)4
E)the number of bonds varies
A)1
B)2
C)3
D)4
E)the number of bonds varies

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
46
Identify the main-group element X that could form the compound 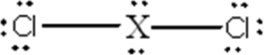
A)C
B)O
C)N
D)F
E)P
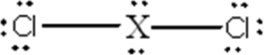
A)C
B)O
C)N
D)F
E)P

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
47
The formula for aluminum chloride is:
A)AlCl
B)AlCl2
C)Al2Cl3
D)AlCl3
E)AlCl4
A)AlCl
B)AlCl2
C)Al2Cl3
D)AlCl3
E)AlCl4

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
48
Draw the Lewis symbol for NaBr.
A)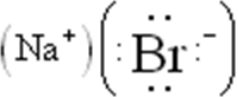
B)Na-Br
C)Na-Br:
D):Na-Br:
E)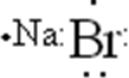
A)
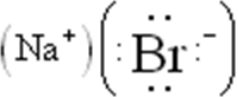
B)Na-Br
C)Na-Br:
D):Na-Br:
E)
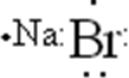

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following statements regarding covalent bonding is incorrect?
A)In covalent bonding, electrons are shared between two atoms.
B)Most elements try to acquire an octet of electrons in their valence shell when bonding.
C)Hydrogen only requires two electrons in its valence shell when bonding.
D)Covalent bonds occur between a metal and a nonmetal.
E)It is possible for two atoms to share more than one pair of electrons.
A)In covalent bonding, electrons are shared between two atoms.
B)Most elements try to acquire an octet of electrons in their valence shell when bonding.
C)Hydrogen only requires two electrons in its valence shell when bonding.
D)Covalent bonds occur between a metal and a nonmetal.
E)It is possible for two atoms to share more than one pair of electrons.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
50
An unknown molecular compound has the following Lewis structure.Which of the following elements could be the identity of X? 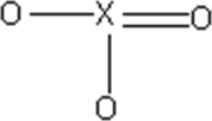
A)Si
B)P
C)S
D)Cl
E)Ne
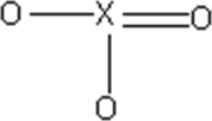
A)Si
B)P
C)S
D)Cl
E)Ne

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
51
The formula for aluminum oxide is:
A)AlO
B)Al2O
C)AlO2
D)Al3O2
E)Al2O3
A)AlO
B)Al2O
C)AlO2
D)Al3O2
E)Al2O3

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
52
Draw the Lewis symbol for CaO. 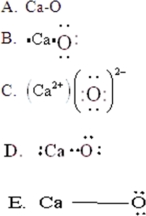
A)A
B)B
C)C
D)D
E)E
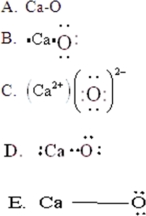
A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
53
Draw the Lewis symbol for K2O. 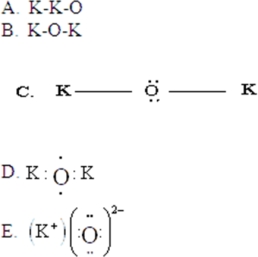
A)A
B)B
C)C
D)D
E)E
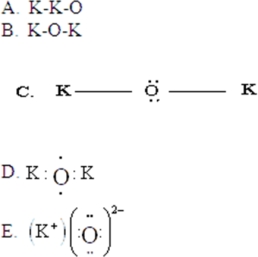
A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following statements regarding ionic compounds is incorrect?
A)In many ionic compounds, a metal accepts electron(s) from a nonmetal.
B)Ionic compounds tend to have high melting points.
C)Electrostatic attractions hold the ions together in an ionic lattice.
D)Ionic compounds must be electrically neutral.
E)The formula of an ionic compound will have the simplest possible whole-number ratio of the ions to each other.
A)In many ionic compounds, a metal accepts electron(s) from a nonmetal.
B)Ionic compounds tend to have high melting points.
C)Electrostatic attractions hold the ions together in an ionic lattice.
D)Ionic compounds must be electrically neutral.
E)The formula of an ionic compound will have the simplest possible whole-number ratio of the ions to each other.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
55
Draw the Lewis symbol for MgCl2.
A)Cl-Mg-Cl
B)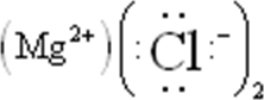
C)Mg-Cl-Cl
D):Cl-Mg-Cl:
E)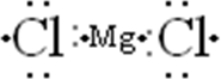
A)Cl-Mg-Cl
B)
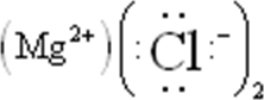
C)Mg-Cl-Cl
D):Cl-Mg-Cl:
E)
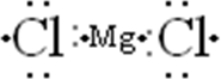

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
56
The formula for calcium sulfide is:
A)Ca2S
B)CaS2
C)CaS
D)Ca2S2
E)Ca2S3
A)Ca2S
B)CaS2
C)CaS
D)Ca2S2
E)Ca2S3

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
57
The formula for calcium nitride is:
A)CaN
B)Ca2N
C)CaN2
D)Ca3N2
E)Ca2N3
A)CaN
B)Ca2N
C)CaN2
D)Ca3N2
E)Ca2N3

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following statements regarding ionic compounds is incorrect?
A)Ions are held together in an ionic lattice by electrostatic attractions.
B)The crystal structure of a given compound will depend on the sizes and number of ions in a formula unit of the compound.
C)Ionic compounds tend to have low melting points.
D)Ionic compounds are formed when a metal and a nonmetal react.
E)In forming an ionic compound, one element transfers one or more electrons to another element.
A)Ions are held together in an ionic lattice by electrostatic attractions.
B)The crystal structure of a given compound will depend on the sizes and number of ions in a formula unit of the compound.
C)Ionic compounds tend to have low melting points.
D)Ionic compounds are formed when a metal and a nonmetal react.
E)In forming an ionic compound, one element transfers one or more electrons to another element.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
59
An unknown molecular compound has the following Lewis structure.Which of the following elements could be the identity of X? 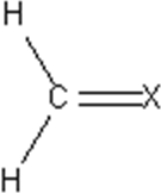
A)C
B)N
C)O
D)Cl
E)Ne

A)C
B)N
C)O
D)Cl
E)Ne

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
60
An unknown molecular compound has the following Lewis structure.Which of the following elements could be the identity of X? 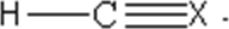
A)S
B)H
C)C
D)Cl
E)N
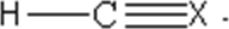
A)S
B)H
C)C
D)Cl
E)N

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
61
Draw the Lewis structure for the NO2- ion.What is the total number of electrons shared between nitrogen and the two oxygen atoms?
A)2
B)3
C)5
D)6
E)8
A)2
B)3
C)5
D)6
E)8

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
62
Which of these molecules or ions has a violation of the octet rule (other than H)?
A)CO
B)H2O
C)PCl5
D)Br2
E)NH3
A)CO
B)H2O
C)PCl5
D)Br2
E)NH3

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following molecules would exhibit resonance?
A)H2S
B)Cl2
C)SO2
D)O2
E)CH4
A)H2S
B)Cl2
C)SO2
D)O2
E)CH4

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following contains a triple bond?
A)H2
B)N2
C)O2
D)F2
E)Cl2
A)H2
B)N2
C)O2
D)F2
E)Cl2

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following molecules would exhibit resonance?
A)H2O
B)N2
C)NO2
D)CO2
E)CCl4
A)H2O
B)N2
C)NO2
D)CO2
E)CCl4

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
66
Which of these molecules or ions has a violation of the octet rule (other than H)?
A)C2H2
B)NH4+
C)SO2
D)BeF2
E)I2
A)C2H2
B)NH4+
C)SO2
D)BeF2
E)I2

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
67
In which of the following does the central atom NOT obey the octet rule?
A)BH3
B)NH3
C)PH3
D)H2S
E)All of these obey the octet rule.
A)BH3
B)NH3
C)PH3
D)H2S
E)All of these obey the octet rule.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
68
How many equivalent resonance structures best represent(s) the NO2- ion?
A)1
B)2
C)3
D)4
E)It does not exhibit resonance.
A)1
B)2
C)3
D)4
E)It does not exhibit resonance.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
69
Which of these molecules or ions has a violation of the octet rule (other than H)?
A)BCl3
B)F2
C)PH3
D)NO3‾
E)SF2
A)BCl3
B)F2
C)PH3
D)NO3‾
E)SF2

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
70
The correctly drawn Lewis formula for CBr4 will have __________.
A)4 single bonds
B)5 single bonds
C)4 single bonds and 1 pair of nonbonding electrons on the carbon atom
D)4 single bonds and 2 pairs of nonbonding electrons on the carbon atom
E)4 single bonds and 3 pairs of nonbonding electrons on the carbon atom
A)4 single bonds
B)5 single bonds
C)4 single bonds and 1 pair of nonbonding electrons on the carbon atom
D)4 single bonds and 2 pairs of nonbonding electrons on the carbon atom
E)4 single bonds and 3 pairs of nonbonding electrons on the carbon atom

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
71
Which of these molecules or ions has a violation of the octet rule (other than H)?
A)CO2
B)H2S
C)SF4
D)CH4
E)PCl3
A)CO2
B)H2S
C)SF4
D)CH4
E)PCl3

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
72
The correctly drawn Lewis formula for C2H2 will have __________.
A)4 single bonds
B)5 single bonds
C)4 single bonds and 1 double bond
D)4 single bonds and 1 triple bond
E)5 double bonds
A)4 single bonds
B)5 single bonds
C)4 single bonds and 1 double bond
D)4 single bonds and 1 triple bond
E)5 double bonds

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following molecules or ions would exhibit resonance?
A)C2H2
B)Br2
C)CO32‾
D)CO
E)SiH4
A)C2H2
B)Br2
C)CO32‾
D)CO
E)SiH4

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
74
Identify the class of organic substance for the molecule CH3CH2-OH.
A)alkane
B)alkene
C)alcohol
D)amine
E)ester
A)alkane
B)alkene
C)alcohol
D)amine
E)ester

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following contains a double bond?
A)CH4
B)C2H6
C)C2H4
D)C2H2
E)H2
A)CH4
B)C2H6
C)C2H4
D)C2H2
E)H2

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
76
The correctly drawn Lewis formula for NH2OH will have __________.
A)3 single bonds and 3 pairs of nonbonding electrons
B)4 single bonds and 3 pairs of nonbonding electrons
C)3 single bonds, 1 double bond, and 2 pairs of nonbonding electrons
D)2 single bonds, 2 double bonds, and 2 pairs of nonbonding electrons
E)4 single bonds and 2 pairs of nonbonding electrons
A)3 single bonds and 3 pairs of nonbonding electrons
B)4 single bonds and 3 pairs of nonbonding electrons
C)3 single bonds, 1 double bond, and 2 pairs of nonbonding electrons
D)2 single bonds, 2 double bonds, and 2 pairs of nonbonding electrons
E)4 single bonds and 2 pairs of nonbonding electrons

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
77
The correctly drawn Lewis formula for SiH4 will have __________.
A)4 single bonds and 1 pair of nonbonding electrons on the Si atom
B)4 double bonds to the Si atom
C)2 single and 2 double bonds on the Si atom
D)2 single bonds to Si and 2 single bonds to terminal H atoms
E)4 single bonds to Si
A)4 single bonds and 1 pair of nonbonding electrons on the Si atom
B)4 double bonds to the Si atom
C)2 single and 2 double bonds on the Si atom
D)2 single bonds to Si and 2 single bonds to terminal H atoms
E)4 single bonds to Si

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following is best represented by two equivalent resonance structures?
A)O3
B)CO2
C)NO3-
D)N2
E)none of these
A)O3
B)CO2
C)NO3-
D)N2
E)none of these

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following contains a triple bond?
A)C2H6
B)HCN
C)NO3-
D)NH3
E)O3
A)C2H6
B)HCN
C)NO3-
D)NH3
E)O3

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
80
The correctly drawn Lewis formula for HCN will have __________.
A)2 single bonds and 5 pairs of nonbonding electrons
B)1 single bond, 1 double bond, and 3 pairs of nonbonding electrons
C)2 double bonds and 2 pairs of nonbonding electrons
D)1 single bond, 1 triple bond, and 1 pair of nonbonding electrons
E)2 double bonds and 1 pair of nonbonding electrons
A)2 single bonds and 5 pairs of nonbonding electrons
B)1 single bond, 1 double bond, and 3 pairs of nonbonding electrons
C)2 double bonds and 2 pairs of nonbonding electrons
D)1 single bond, 1 triple bond, and 1 pair of nonbonding electrons
E)2 double bonds and 1 pair of nonbonding electrons

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck



