Deck 6: Quantities in Chemical Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/120
Play
Full screen (f)
Deck 6: Quantities in Chemical Reactions
1
Consider the reaction between hydrogen and oxygen gases to form water: 2H2(g) + O2(g) 2H2O(l) Which of the following is not conserved in this reaction?
A)atoms
B)moles of atoms
C)moles of molecules
D)mass
A)atoms
B)moles of atoms
C)moles of molecules
D)mass
moles of molecules
2
Consider the reaction between acetylene, C2H2, and oxygen in a welding torch: 2C2H2(g) + 5O2(g) 4CO2(g) + 2H2O(g) Which of the following is not conserved in this reaction?
A)atoms
B)moles of atoms
C)moles of molecules
D)mass
A)atoms
B)moles of atoms
C)moles of molecules
D)mass
moles of molecules
3
Consider the reaction between sodium metal and chlorine gas to form sodium chloride: 2Na(s) + Cl2(g) 2NaCl(s) Which of the following is not conserved in this reaction?
A)atoms
B)moles of atoms
C)moles of molecules
D)mass
A)atoms
B)moles of atoms
C)moles of molecules
D)mass
moles of molecules
4
When the mixture of molecules shown in the molecular-level image undergoes complete reaction, all of these molecules are converted to products.Which of the following reactions could this represent? 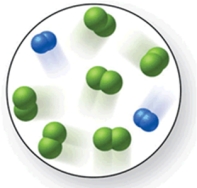
A)2N2 + 3O2 2N2O3
B)N2 + 2Cl2 N2Cl4
C)O2 + 2H2 2H2O
D)N2 + 3Cl2 2NCl3
E)3N2 + 2H2 3N2H4

A)2N2 + 3O2 2N2O3
B)N2 + 2Cl2 N2Cl4
C)O2 + 2H2 2H2O
D)N2 + 3Cl2 2NCl3
E)3N2 + 2H2 3N2H4

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
5
When mixed, solutions of aluminum nitrate, Al(NO3)3, and ammonium carbonate, (NH4)2CO3, will form a precipitate of aluminum carbonate, Al2(CO3)3.The balanced equation is 2Al(NO3)3(aq) + 3(NH4)2CO3(aq) Al2(CO3)3 (s) + 6NH4NO3(aq) Which of the following statements regarding this reaction is incorrect?
A)2 moles of Al(NO3)3 will react with 3 moles of (NH4)2CO3.
B)If 6 moles of (NH4)2CO3 react with sufficient Al(NO3)3, 2 moles of Al2(CO3)3 should form.
C)If 0.5 mole of (NH4)2CO3 react with sufficient Al(NO3)3, 3 moles of Al2(CO3)3 should form.
D)If 1.5 moles of Al2(CO3)3 are formed, given sufficient starting materials, then 9 moles of NH4NO3 should also form.
E)4 moles of Al(NO3)3 will react with 6 moles of (NH4)2CO3.
A)2 moles of Al(NO3)3 will react with 3 moles of (NH4)2CO3.
B)If 6 moles of (NH4)2CO3 react with sufficient Al(NO3)3, 2 moles of Al2(CO3)3 should form.
C)If 0.5 mole of (NH4)2CO3 react with sufficient Al(NO3)3, 3 moles of Al2(CO3)3 should form.
D)If 1.5 moles of Al2(CO3)3 are formed, given sufficient starting materials, then 9 moles of NH4NO3 should also form.
E)4 moles of Al(NO3)3 will react with 6 moles of (NH4)2CO3.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is the best (simplest) balanced equation to represent the chemical reaction shown in the figure on any scale? 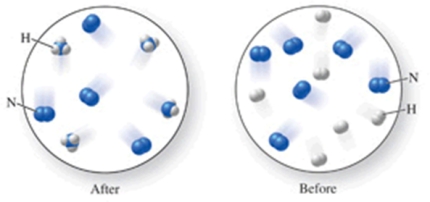
A)12N + 12H 12NH
B)6N2 + 6H2 4NH3
C)6N2 + 6H2 4NH3 + 4N2
D)12N + 12H 4NH3 + 8N
E)N2 + 3H2 2NH3

A)12N + 12H 12NH
B)6N2 + 6H2 4NH3
C)6N2 + 6H2 4NH3 + 4N2
D)12N + 12H 4NH3 + 8N
E)N2 + 3H2 2NH3

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
7
When acetylene, C2H2, a fuel used in welding, is burned in air, two molecules of acetylene combine with five oxygen molecules to form four CO2 molecules and two H2O molecules.Select the statement below that is incorrect in regard to this reaction.
A)The balanced equation for the reaction is 2C2H2 + 5O2 4CO2 + 2H2O.
B)If 8 acetylene molecules react, 12 CO2 molecules should form.
C)If 12 H2O molecules are formed, then 30 O2 molecules must have reacted.
D)If 12 CO2 molecules are formed, then 6 H2O molecules should also form.
E)If 24 ethanol molecules react, then 60 oxygen molecules must also react
A)The balanced equation for the reaction is 2C2H2 + 5O2 4CO2 + 2H2O.
B)If 8 acetylene molecules react, 12 CO2 molecules should form.
C)If 12 H2O molecules are formed, then 30 O2 molecules must have reacted.
D)If 12 CO2 molecules are formed, then 6 H2O molecules should also form.
E)If 24 ethanol molecules react, then 60 oxygen molecules must also react

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is the best (simplest) balanced equation to represent the chemical reaction shown in the figure on any scale? 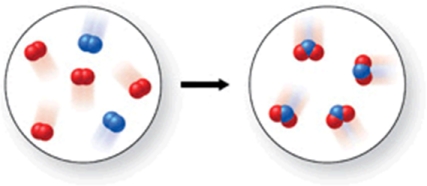
A)4A + 8B 12AB
B)2A2 + 4B2 4A2B
C)2A2 + 4B2 4AB2
D)A2 + B2 AB2
E)A2 + 2B2 2AB2

A)4A + 8B 12AB
B)2A2 + 4B2 4A2B
C)2A2 + 4B2 4AB2
D)A2 + B2 AB2
E)A2 + 2B2 2AB2

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following equations is balanced?
A)P4(s) + 10O2(g) P4O10(s)
B)ZnS(s) + 3O2(g) ZnO(s) + 2SO2(g)
C)NH3(g) + O2(g) NO2(g) + H2O(g)
D)4KBrO3(s) 3KBrO4(s) + KBr(s)
E)2Na(s) + P(s) Na3P(s)
A)P4(s) + 10O2(g) P4O10(s)
B)ZnS(s) + 3O2(g) ZnO(s) + 2SO2(g)
C)NH3(g) + O2(g) NO2(g) + H2O(g)
D)4KBrO3(s) 3KBrO4(s) + KBr(s)
E)2Na(s) + P(s) Na3P(s)

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
10
When ammonium carbonate, (NH4)2CO3, dissolves in water, the ions that are formed for each formula unit that dissolves are:
A)(NH4)22+(aq) + CO32-(aq)
B)2NH4+(aq) + CO32-(aq)
C)2N3-(aq) + 4H+(aq) + CO32-(aq)
D)2N3-(aq) + 4H+(aq) + C(s) + O32-(aq)
E)2N3-(aq) + 4H+(aq) + C4+(aq)+ 3O2-(aq)
A)(NH4)22+(aq) + CO32-(aq)
B)2NH4+(aq) + CO32-(aq)
C)2N3-(aq) + 4H+(aq) + CO32-(aq)
D)2N3-(aq) + 4H+(aq) + C(s) + O32-(aq)
E)2N3-(aq) + 4H+(aq) + C4+(aq)+ 3O2-(aq)

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
11
When mixed, solutions of silver nitrate, AgNO3, and sodium phosphate, Na3PO4, will form a precipitate of silver phosphate, Ag3PO4.The balanced equation is 3AgNO3(aq) + Na3PO4(aq) Ag3PO4(s) + 3NaNO3(aq) Which of the following statements regarding this reaction is incorrect?
A)6 moles of AgNO3 will react with 2 moles of Na3PO4.
B)9 moles of AgNO3 should react to form 2 moles of Ag3PO4, given sufficient Na3PO4.
C)1.5 moles of NaNO3 should form when 0.5 mole of Na3PO4 reacts with sufficient AgNO3.
D)3 moles of Ag3PO4 should form when 3 moles of Na3PO4 react with sufficient AgNO3.
E)2 moles of Na3PO4 will react with 6 moles of AgNO3.
A)6 moles of AgNO3 will react with 2 moles of Na3PO4.
B)9 moles of AgNO3 should react to form 2 moles of Ag3PO4, given sufficient Na3PO4.
C)1.5 moles of NaNO3 should form when 0.5 mole of Na3PO4 reacts with sufficient AgNO3.
D)3 moles of Ag3PO4 should form when 3 moles of Na3PO4 react with sufficient AgNO3.
E)2 moles of Na3PO4 will react with 6 moles of AgNO3.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
12
All of the following may change during a chemical reaction except
A)the total number of atoms in the system.
B)the temperature of the system.
C)the color of the system.
D)the total number of molecules in the system.
E)the physical state of the system.
A)the total number of atoms in the system.
B)the temperature of the system.
C)the color of the system.
D)the total number of molecules in the system.
E)the physical state of the system.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
13
When mixed, solutions of copper(II) nitrate, Cu(NO3)2, and sodium phosphate, Na3PO4, will form a precipitate of copper phosphate, Cu3(PO4)2.The balanced equation is 3Cu(NO3)2(aq) + 2Na3PO4(aq) Cu3(PO4)2(s) + 6NaNO3(aq) Which of the following statements regarding this reaction is incorrect?
A)8 moles of Na3PO4 will react with 12 moles of Cu(NO3)2.
B)1 mole of NaNO3 should form when 0.5 mole of Cu(NO3)2 reacts with sufficient Na3PO4.
C)12 moles of Cu3(PO4)2 should form when 36 moles of Cu(NO3)2 reacts with sufficient Na3PO4.
D)If 10 moles of Na3PO4 react with sufficient Cu(NO3)2, 4 moles of Cu3(PO4)2 should form.
E)If 5 moles of Cu3(PO4)2 are needed, it would require the combination of 15 moles of Cu(NO3)2 and 10 moles of Na3PO4.
A)8 moles of Na3PO4 will react with 12 moles of Cu(NO3)2.
B)1 mole of NaNO3 should form when 0.5 mole of Cu(NO3)2 reacts with sufficient Na3PO4.
C)12 moles of Cu3(PO4)2 should form when 36 moles of Cu(NO3)2 reacts with sufficient Na3PO4.
D)If 10 moles of Na3PO4 react with sufficient Cu(NO3)2, 4 moles of Cu3(PO4)2 should form.
E)If 5 moles of Cu3(PO4)2 are needed, it would require the combination of 15 moles of Cu(NO3)2 and 10 moles of Na3PO4.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
14
When potassium carbonate, K2CO3, dissolves in water, the ions that are formed for each formula unit that dissolves are:
A)K22+(aq) + CO32-(aq)
B)2K2+(aq) + CO32-(aq)
C)2K+(aq) + CO32-(aq)
D)2K+(aq) + C(s) + O32-(aq)
E)K22+(aq) + C4+(aq) + 3O2-(aq)
A)K22+(aq) + CO32-(aq)
B)2K2+(aq) + CO32-(aq)
C)2K+(aq) + CO32-(aq)
D)2K+(aq) + C(s) + O32-(aq)
E)K22+(aq) + C4+(aq) + 3O2-(aq)

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following is the best (simplest) balanced equation to represent the chemical reaction shown in the figure on any scale? 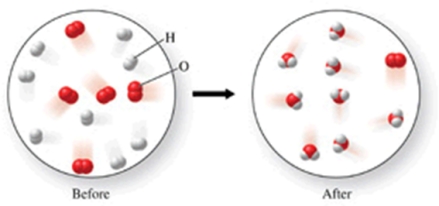
A)16H + 10O 16H + 10O
B)16H + 10O 8H2O + O2
C)8H2 + 5O2 8H2O + O2
D)2H2 + O2 2H2O
E)4H2 + 2O2 4H2O

A)16H + 10O 16H + 10O
B)16H + 10O 8H2O + O2
C)8H2 + 5O2 8H2O + O2
D)2H2 + O2 2H2O
E)4H2 + 2O2 4H2O

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
16
When the mixture of molecules shown in the molecular-level image undergoes complete reaction, all of these molecules are converted to products.Which of the following reactions could this represent? 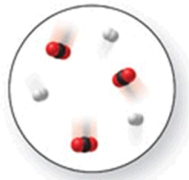
A)N2 + O2 2NO
B)N2 + 2Cl2 N2Cl4
C)N2 + 2O2 2NO2
D)N2 + 3H2 2NH3
E)N2 + 3O2 2NO3

A)N2 + O2 2NO
B)N2 + 2Cl2 N2Cl4
C)N2 + 2O2 2NO2
D)N2 + 3H2 2NH3
E)N2 + 3O2 2NO3

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
17
Phosphine, PH3, a reactive and poisonous compound, reacts with oxygen as follows: 4PH3(g) + 8O2(g) P4O10(s) + 6H2O(g) If 9.2 moles of phosphine react with sufficient oxygen, how many moles of P4O10 should form?
A)4.0 moles
B)9.2 moles
C)37 moles
D)2.3 moles
E)6.0 moles
A)4.0 moles
B)9.2 moles
C)37 moles
D)2.3 moles
E)6.0 moles

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
18
When one molecule of propane, C3H8, burns in a gas grill, it combines with five oxygen molecules to form three CO2 molecules and four H2O molecules.Select the statement below that is incorrect in regard to this reaction.
A)The balanced equation for the reaction is C3H8 + 5O2 3CO2 + 4H2O.
B)If 5 propane molecules react, 15 CO2 molecules should form.
C)If 5 propane molecules react, 25 O2 molecules must also react.
D)If 15 O2 molecules react, 9 H2O molecules should form.
E)If 12 CO2 molecules are formed, then 4 propane molecules must have reacted.
A)The balanced equation for the reaction is C3H8 + 5O2 3CO2 + 4H2O.
B)If 5 propane molecules react, 15 CO2 molecules should form.
C)If 5 propane molecules react, 25 O2 molecules must also react.
D)If 15 O2 molecules react, 9 H2O molecules should form.
E)If 12 CO2 molecules are formed, then 4 propane molecules must have reacted.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
19
When sodium sulfate, Na2SO4, dissolves in water, the ions that are formed for each formula unit that dissolves are:
A)Na22+(aq) + SO42-(aq)
B)2Na2+(aq) + SO42-(aq)
C)2Na+(aq) + SO42-(aq)
D)Na22+(aq) + S(s) + O42-(aq)
E)Na22+(aq) + S2-(aq) + 4O2-(aq)
A)Na22+(aq) + SO42-(aq)
B)2Na2+(aq) + SO42-(aq)
C)2Na+(aq) + SO42-(aq)
D)Na22+(aq) + S(s) + O42-(aq)
E)Na22+(aq) + S2-(aq) + 4O2-(aq)

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
20
When ethanol, C2H5OH, a component in some gasoline mixtures, is burned in air, one molecule of ethanol combines with three oxygen molecules to form two CO2 molecules and three H2O molecules.Select the statement below that is incorrect in regard to this reaction.
A)The balanced equation for the reaction is C2H5OH + 3O2 2CO2 + 3H2O.
B)If 4 ethanol molecules react, 8 CO2 molecules should form.
C)If 12 H2O molecules are formed, then 9 O2 molecules must have reacted.
D)If 15 ethanol molecules react, then 45 oxygen molecules must also react.
E)If 12 CO2 molecules are formed, then 18 H2O molecules should also form.
A)The balanced equation for the reaction is C2H5OH + 3O2 2CO2 + 3H2O.
B)If 4 ethanol molecules react, 8 CO2 molecules should form.
C)If 12 H2O molecules are formed, then 9 O2 molecules must have reacted.
D)If 15 ethanol molecules react, then 45 oxygen molecules must also react.
E)If 12 CO2 molecules are formed, then 18 H2O molecules should also form.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
21
Given the balanced equation 4NH3(g) + 5O2(g) 4NO(g) + 6H2O(g), if 32.5 g of NH3 react with sufficient oxygen, how many grams of H2O should form?
A)51.6 g
B)8.60 g
C)34.4 g
D)206.4 g
E)878 g
A)51.6 g
B)8.60 g
C)34.4 g
D)206.4 g
E)878 g

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
22
When mercury(II) oxide, a red crystalline solid, is heated, it decomposes to form liquid mercury and oxygen gas, according to the following equation: ___HgO(s) ___Hg(l) + ___O2(g) (unbalanced) Balance the equation and determine the mass of mercury that should be formed when 15.6 g of HgO is heated.
A)28.9 g
B)7.22 g
C)14.4 g
D)16.9 g
E)13.2 g
A)28.9 g
B)7.22 g
C)14.4 g
D)16.9 g
E)13.2 g

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
23
Consider the reaction between sodium metal and chlorine gas to form sodium chloride (table salt): 2Na(s) + Cl2(g) 2NaCl(s) If 12.5 g of sodium react with sufficient chlorine, how many grams of sodium chloride should form?
A)15.9 g
B)31.8 g
C)63.6 g
D)4.92 g
E)9.84 g
A)15.9 g
B)31.8 g
C)63.6 g
D)4.92 g
E)9.84 g

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
24
Small amounts of oxygen gas can be produced for laboratory use by heating potassium chlorate, which causes it to decompose by the following reaction: ___KClO3(s) ____KCl(s) + ___O2(g) (unbalanced) Balance the equation, and determine the mass of oxygen that should be formed if 10.0 g of potassium chlorate decomposes.
A)7.83 g
B)115 g
C)3.92 g
D)38.3 g
E)57.4 g
A)7.83 g
B)115 g
C)3.92 g
D)38.3 g
E)57.4 g

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
25
When copper reacts with sulfur at high temperature, copper(I) sulfide is formed. 2Cu(s) + S(s) Cu2S(s) If the mass of the Cu2S formed is 1.17 g, what mass of copper should have reacted?
A)0.934 g
B)not enough information
C)0.78 g
D)2.34 g
E)0.467 g
A)0.934 g
B)not enough information
C)0.78 g
D)2.34 g
E)0.467 g

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
26
When magnesium is heated in air, it reacts with oxygen to form magnesium oxide: 2Mg(s) + O2(g) 2MgO(s) If the mass of the magnesium solid increases by 0.335 g, what mass of magnesium metal should have reacted?
A)0.882 g
B)0.441 g
C)0.509 g
D)1.02 g
E)not enough information
A)0.882 g
B)0.441 g
C)0.509 g
D)1.02 g
E)not enough information

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
27
Consider the reaction between hydrogen and oxygen gases to form water: 2H2(g) + O2(g) 2H2O(l) If 8.5 moles of oxygen react with sufficient hydrogen, how many grams of water should form?
A)0.94 g
B)0.47 g
C)76 g
D)3.1 x 102 g
E)1.5 x 102 g
A)0.94 g
B)0.47 g
C)76 g
D)3.1 x 102 g
E)1.5 x 102 g

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
28
Consider the reaction between acetylene, C2H2, and oxygen in a welding torch: 2C2H2(g) + 5O2(g) 4CO2(g) + 2H2O(g) If 5.4 moles of acetylene react with sufficient oxygen, how many grams of CO2 should form?
A)2.4 x 102 g
B)9.5 x 102 g
C)4.8 x 102 g
D)1.5 x 102 g
E)0.49 g
A)2.4 x 102 g
B)9.5 x 102 g
C)4.8 x 102 g
D)1.5 x 102 g
E)0.49 g

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
29
Given that 4NH3(g) + 5O2(g) 4NO(g) + 6H2O(g), if 4.5 moles of NH3 react with sufficient oxygen, how many moles of H2O should form?
A)4.0
B)4.5
C)6.0
D)6.8
E)5.5
A)4.0
B)4.5
C)6.0
D)6.8
E)5.5

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
30
Phosphine, PH3, a reactive and poisonous compound, reacts with oxygen as follows: 4PH3(g) + 8O2(g) P4O10(s) + 6H2O(g) If you need to make 6.5 moles of P4O10 , how many moles of PH3 is required for the reaction?
A)6.5 moles
B)13 moles
C)26 moles
D)3.2 moles
E)1.6 moles
A)6.5 moles
B)13 moles
C)26 moles
D)3.2 moles
E)1.6 moles

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
31
Consider the reaction between sodium metal and chlorine gas to form sodium chloride (table salt): 2Na(s) + Cl2(g) 2NaCl(s) If 3.6 moles of chlorine react with sufficient sodium, how many grams of sodium chloride should form?
A)1.1 x 102 g
B)2.1 x 102 g
C)4.2 x 102 g
D)0.13 g
E)0.062 g
A)1.1 x 102 g
B)2.1 x 102 g
C)4.2 x 102 g
D)0.13 g
E)0.062 g

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
32
Given that 4NH3(g) + 5O2(g) 4NO(g) + 6H2O(g), if 8.2 moles of NH3 react with sufficient oxygen, how many moles of water should form?
A)6.0 moles
B)4.0 moles
C)5.0 moles
D)12 moles
E)8.2 moles
A)6.0 moles
B)4.0 moles
C)5.0 moles
D)12 moles
E)8.2 moles

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
33
Phosphine, PH3, a reactive and poisonous compound, reacts with oxygen as follows: 4PH3(g) + 8O2(g) P4O10(s) + 6H2O(g) If 15.0 g of phosphine reacts with sufficient oxygen, how many grams of P4O10 will be formed?
A)125 g
B)31.3 g
C)5.00 x 102 g
D)18.9 g
E)75.7 g
A)125 g
B)31.3 g
C)5.00 x 102 g
D)18.9 g
E)75.7 g

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
34
Given that 4NH3(g) + 5O2(g) 4NO(g) + 6H2O(g), if 6.3 moles of NH3 react with sufficient oxygen, how many moles of NO should form?
A)4.0 moles
B)6.3 moles
C)6.0 moles
D)5.0 moles
E)3.2 moles
A)4.0 moles
B)6.3 moles
C)6.0 moles
D)5.0 moles
E)3.2 moles

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
35
Given the balanced equation 4NH3(g) + 5O2(g) 4NO(g) + 6H2O(g), if 82.0 g of NH3 react with sufficient oxygen, how many grams of NO should form?
A)145 g
B)5.80 x 102 g
C)46.5 g
D)186 g
E)11.6 g
A)145 g
B)5.80 x 102 g
C)46.5 g
D)186 g
E)11.6 g

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
36
When mercury(II) oxide, a red crystalline solid, is heated, it decomposes to form liquid mercury and oxygen gas, according to the following equation: ___HgO(s) ___Hg(l) + ___O2(g) (unbalanced) Balance the equation and determine the mass of mercury that should be formed when 12.3 g of HgO is heated.
A)22.8 g
B)11.4 g
C)5.70 g
D)13.3 g
E)6.64 g
A)22.8 g
B)11.4 g
C)5.70 g
D)13.3 g
E)6.64 g

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
37
When phosphorus reacts with chlorine, phosphorus trichloride is formed according to the equation: ___P4(s) + ___Cl2(g) ___PCl3(l) (unbalanced) Balance the equation and determine how many grams of chlorine would be required to react with 10.6 g of phosphorus.
A)6.07 g
B)24.3 g
C)36.4 g
D)18.5 g
E)74.1 g
A)6.07 g
B)24.3 g
C)36.4 g
D)18.5 g
E)74.1 g

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
38
When a 0.525 g piece of zinc is placed in a solution of copper(II) sulfate, copper metal and zinc sulfate are formed.Balance the equation for the reaction, and determine the mass of copper(II) sulfate that would react with this quantity of zinc. ___ Zn(s) + ___CuSO4(aq) ___ZnSO4(aq) + ___Cu(s) (unbalanced)
A)65.4 g
B)0.641 g
C)1.28 g
D)2.56 g
E)159 g
A)65.4 g
B)0.641 g
C)1.28 g
D)2.56 g
E)159 g

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
39
Consider the reaction between sodium metal and chlorine gas to form sodium chloride (table salt): 2Na(s) + Cl2(g) 2NaCl(s) If the mass of the sodium solid increases by 0.500 g, what mass of sodium metal should have reacted?
A)0.250 g
B)0.500 g
C)0.0811 g
D)0.162 g
E)0.324 g
A)0.250 g
B)0.500 g
C)0.0811 g
D)0.162 g
E)0.324 g

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
40
Small amounts of oxygen gas can be produced for laboratory use by heating potassium chlorate, which causes it to decompose by the following reaction: ___KClO3(s) ____KCl(s) + ___O2(g) (unbalanced) Balance the equation, and determine the mass of oxygen that should be formed if 15.0 g of potassium chlorate decomposes.
A)11.7 g
B)57.5 g
C)173 g
D)5.88 g
E)86.1 g
A)11.7 g
B)57.5 g
C)173 g
D)5.88 g
E)86.1 g

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
41
When phosphorus reacts with chlorine, phosphorus trichloride is formed according to the following equation: ___P4(s) + ___Cl2(g) ___PCl3(l) (unbalanced) Balance the equation and determine how many grams of phosphorus reactant would be required to produce 75.0 g of phosphorus trichloride, assuming there is sufficient chlorine available.
A)67.8 g
B)135 g
C)16.9 g
D)271 g
E)333 g
A)67.8 g
B)135 g
C)16.9 g
D)271 g
E)333 g

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
42
Ammonia is usually made by the following reaction: N2(g) + 3H2(g) 2NH3(g) What is the maximum amount of ammonia that can be formed if 25 molecules of nitrogen are mixed with 60 molecules of hydrogen?
A)20 molecules
B)25 molecules
C)30 molecules
D)40 molecules
E)85 molecules
A)20 molecules
B)25 molecules
C)30 molecules
D)40 molecules
E)85 molecules

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
43
Phosphorus trichloride can be made by the following reaction: P4(s) + 6Cl2(g) 4PCl3(l) What is the maximum amount of phosphorus trichloride that can be formed if 15 molecules of P4 are mixed with 42 molecules of chlorine?
A)4 molecules
B)12 molecules
C)24 molecules
D)28 molecules
E)57 molecules
A)4 molecules
B)12 molecules
C)24 molecules
D)28 molecules
E)57 molecules

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
44
If you have eight bicycle wheels and five frames, how many bikes could you build (assuming that each bike requires one frame and two wheels), and what would be left over?
A)Four bikes could be built, and nothing would be left over.
B)Four bikes could be built, and one frame would be left over.
C)Five bikes could be built, and three wheels would be left over.
D)Five bikes could be built, and nothing would be left over.
E)Three bikes could be built, and two frames would be left over.
A)Four bikes could be built, and nothing would be left over.
B)Four bikes could be built, and one frame would be left over.
C)Five bikes could be built, and three wheels would be left over.
D)Five bikes could be built, and nothing would be left over.
E)Three bikes could be built, and two frames would be left over.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
45
Nitrogen and hydrogen react together to form ammonia according to the following equation: ___N2(g) + ___H2(g) ___NH3(g) (unbalanced) Balance the equation, and determine how many grams of hydrogen reactant would be required to produce 50.0 g of ammonia, assuming there is sufficient nitrogen available.
A)4.46 g
B)5.94 g
C)4.81 g
D)8.91 g
E)not enough information
A)4.46 g
B)5.94 g
C)4.81 g
D)8.91 g
E)not enough information

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
46
Consider the following reaction: Cr2O3(s) + 3CCl4(l) 2CrCl3(s) + 3COCl2(g) green colorless purple colorless solid liquid solid gas When the green solid is mixed with the colorless liquid, the mixture starts to bubble and fume.When all action has stopped, a dry purple solid containing solid green specks remains.Which substance is the limiting reactant?
A)Cr2O3
B)CCl4
C)CrCl3
D)COCl2
E)there is no limiting reactant
A)Cr2O3
B)CCl4
C)CrCl3
D)COCl2
E)there is no limiting reactant

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
47
Ammonia is usually made by the following reaction: N2(g) + 3H2(g) 2NH3(g) What is the maximum amount of ammonia that can be formed if 30 molecules of nitrogen are mixed with 100 molecules of hydrogen?
A)20 molecules
B)30 molecules
C)40 molecules
D)60 molecules
E)70 molecules
A)20 molecules
B)30 molecules
C)40 molecules
D)60 molecules
E)70 molecules

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
48
A pamphlet requires one cover, 14 pieces of white paper, and three sheets of colored paper.How many pamphlets could be made, and what would be left over, if there are 50 covers, 500 sheets of white paper, and 100 sheets of colored paper available?
A)33 pamphlets could be made, and one sheet of colored paper, 38 sheets of white paper, and 17 covers would be left over.
B)50 pamphlets could be made, and there would be no leftovers.
C)650 pamphlets could be made, and there would be no leftovers.
D)34 pamphlets could be made, and 24 sheets of white paper and 16 covers would be left over.
E)35 pamphlets could be made, and 10 sheets of white paper and 15 covers would be left over.
A)33 pamphlets could be made, and one sheet of colored paper, 38 sheets of white paper, and 17 covers would be left over.
B)50 pamphlets could be made, and there would be no leftovers.
C)650 pamphlets could be made, and there would be no leftovers.
D)34 pamphlets could be made, and 24 sheets of white paper and 16 covers would be left over.
E)35 pamphlets could be made, and 10 sheets of white paper and 15 covers would be left over.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
49
Nitrogen and hydrogen react together to form ammonia according to the equation: ___N2(g) + ___H2(g) ___NH3(g) (unbalanced) Balance the equation, and determine how many grams of hydrogen would be required to react with 25.2 g of nitrogen.
A)1.82 g
B)3.64 g
C)5.45 g
D)1.34 g
E)0.891
A)1.82 g
B)3.64 g
C)5.45 g
D)1.34 g
E)0.891

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
50
If you wish to make sandwiches which consist of two slices of bread, one ham slice, and three pickle slices, how many sandwiches could you make if you have 12 slices of bread, five slices of ham, and 20 pickle slices, and what would be left over?
A)Six sandwiches could be made, and two pickle slices would be left over.
B)Four sandwiches could be made, and four slices of bread and eight pickle slices would be left over.
C)Five sandwiches could be made, and two slices of bread and five pickle slices would be left over.
D)Twelve sandwiches could be made, and there would be no leftovers.
E)Five sandwiches could be made, and two slices of bread and no pickle slices would be left over.
A)Six sandwiches could be made, and two pickle slices would be left over.
B)Four sandwiches could be made, and four slices of bread and eight pickle slices would be left over.
C)Five sandwiches could be made, and two slices of bread and five pickle slices would be left over.
D)Twelve sandwiches could be made, and there would be no leftovers.
E)Five sandwiches could be made, and two slices of bread and no pickle slices would be left over.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
51
When 5.0 g CaCl2 is dissolved in enough water to make a 0.500 L solution, what is the molarity of ions in solution?
A)0.135 M
B)0.045 M
C)0.270 M
D)10.0 M
E)2.50 M
A)0.135 M
B)0.045 M
C)0.270 M
D)10.0 M
E)2.50 M

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
52
Nitrogen and hydrogen react together to form ammonia according to the equation: ___N2(g) + ___H2(g) ___NH3(g) (unbalanced) Balance the equation, and determine how many grams of hydrogen would be required to react with 50.4 g of nitrogen.
A)10.9 g
B)3.64 g
C)7.28 g
D)2.68 g
E)1.78 g
A)10.9 g
B)3.64 g
C)7.28 g
D)2.68 g
E)1.78 g

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
53
When phosphorus reacts with chlorine, phosphorus trichloride is formed according to the equation: ___P4(s) + ___Cl2(g) ___PCl3(l) (unbalanced) Balance the equation and determine how many grams of phosphorus reactant would be required to produce 25.0 g of phosphorus trichloride, assuming there is sufficient chlorine available.
A)22.6 g
B)45.1 g
C)5.64 g
D)111 g
E)90.2 g
A)22.6 g
B)45.1 g
C)5.64 g
D)111 g
E)90.2 g

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
54
Nitrogen and hydrogen react together to form ammonia according to the equation: ___N2(g) + ___H2(g) ___NH3(g) (unbalanced) Balance the equation, and determine how many grams of hydrogen reactant would be required to produce 25.0 g of ammonia, assuming there is sufficient nitrogen available.
A)2.23 g
B)4.46 g
C)2.97 g
D)2.40 g
E)not enough information
A)2.23 g
B)4.46 g
C)2.97 g
D)2.40 g
E)not enough information

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
55
When phosphorus reacts with chlorine, phosphorus trichloride is formed according to the following equation: ___P4(s) + ___Cl2(g) ___PCl3(l) (unbalanced) Balance the equation and determine how many grams of chlorine would be required to react with 21.2 g of phosphorus.
A)12.1 g
B)48.6 g
C)37.0 g
D)148 g
E)72.8 g
A)12.1 g
B)48.6 g
C)37.0 g
D)148 g
E)72.8 g

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
56
Consider the following reaction: CrCl3(s) + KCl(s) + 2H2SO4(l) KCr(SO4)2(s) + 4HCl(g) green white colorless purple colorless solid solid liquid solid gas When the green solid is mixed with the white solid and the colorless liquid is added, the mixture starts to bubble and fume.When all action has stopped, a wet purple solid containing solid white specks remains.Which substance is the limiting reactant?
A)CrCl3
B)KCl
C)H2SO4
D)KCr(SO4)2
E)HCl
A)CrCl3
B)KCl
C)H2SO4
D)KCr(SO4)2
E)HCl

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
57
When 5.0 g CaCl2 is dissolved in water, how many moles of ions are in solution?
A)0.135 mole
B)0.045 mole
C)5.0 moles
D)15.0 moles
E)1660 moles
A)0.135 mole
B)0.045 mole
C)5.0 moles
D)15.0 moles
E)1660 moles

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
58
Consider the following reaction: Cr2O3(s) + 3CCl4(l) 2CrCl3(s) + 3COCl2(g) green colorless purple colorless solid liquid solid gas When the green solid is mixed with the colorless liquid, the mixture starts to bubble and fume.When all action has stopped, a wet purple solid remains.Which substance is the limiting reactant?
A)Cr2O3
B)CCl4
C)CrCl3
D)COCl2
E)there is no limiting reactant
A)Cr2O3
B)CCl4
C)CrCl3
D)COCl2
E)there is no limiting reactant

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
59
Phosphorus trichloride can be made by the following reaction: P4(s) + 6Cl2(g) 4PCl3(l) What is the maximum amount of phosphorus trichloride that can be formed if 10 molecules of P4 are mixed with 36 molecules of chlorine?
A)4 molecules
B)6 molecules
C)12 molecules
D)24 molecules
E)46 molecules
A)4 molecules
B)6 molecules
C)12 molecules
D)24 molecules
E)46 molecules

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
60
When 3.0 mol CaCl2 dissolves in water, how many moles of ions are in solution?
A)1.0 mole
B)3.0 moles
C)6.0 moles
D)9.0 moles
E)12 moles
A)1.0 mole
B)3.0 moles
C)6.0 moles
D)9.0 moles
E)12 moles

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
61
Consider the following reaction: 3NO2(g) + H2O(l) 2HNO3(l) + NO(g) How many moles of NO2 are required to react with 1.50 moles of H2O to form 3.00 moles of HNO3?
A)1.50 mol
B)3.00 mol
C)4.00 mol
D)4.50 mol
E)9.00 mol
A)1.50 mol
B)3.00 mol
C)4.00 mol
D)4.50 mol
E)9.00 mol

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
62
The figure shows a molecular-level diagram of reactant molecules for the reaction: 2C2H2(g) + 5O2(g) 4CO2(g) + 2H2O(g) 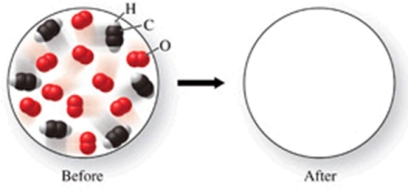 List the number and formulas of the molecules that should be present after the reaction takes place.
List the number and formulas of the molecules that should be present after the reaction takes place.
A)4CO2 + 2H2O
B)4CO2 + 2H2O + 2C2H2
C)4CO2 + 2H2O + 2C2H2 + 5O2
D)6CO2 + 3H2O + 3O2
E)8CO2 + 4H2O + 2C2H2
 List the number and formulas of the molecules that should be present after the reaction takes place.
List the number and formulas of the molecules that should be present after the reaction takes place.A)4CO2 + 2H2O
B)4CO2 + 2H2O + 2C2H2
C)4CO2 + 2H2O + 2C2H2 + 5O2
D)6CO2 + 3H2O + 3O2
E)8CO2 + 4H2O + 2C2H2

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
63
Aluminum metal reacts with sulfuric acid according to the following equation: 2Al(s) + 3H2SO4(aq) Al2(SO4)3(s) + 3H2(g) If 12.9 g of aluminum reacts with excess sulfuric acid, and 62.4 g of Al2(SO4)3 are collected, what is the percent yield of Al2(SO4)3?
A)81.8%
B)49.5%
C)76.3%
D)131%
E)not enough information given
A)81.8%
B)49.5%
C)76.3%
D)131%
E)not enough information given

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
64
Aluminum metal reacts with sulfuric acid according to the following equation: 2Al(s) + 3H2SO4(aq) Al2(SO4)3(s) + 3H2(g) If 10.0 g of aluminum reacts with excess sulfuric acid, and 54.2 g of Al2(SO4)3 are collected, what is the percent yield of Al2(SO4)3?
A)63.4%
B)85.5%
C)117%
D)47.1%
E)not enough information given
A)63.4%
B)85.5%
C)117%
D)47.1%
E)not enough information given

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
65
Aluminum reacts with oxygen according to the following reaction: 4Al(s) + 3O2(g) 2Al2O3(s) If 24 moles of aluminum are combined with 12 moles of oxygen, how many moles of Al2O3 should form?
A)4.0 moles
B)8.0 moles
C)6.0 moles
D)5.0 moles
E)26 moles
A)4.0 moles
B)8.0 moles
C)6.0 moles
D)5.0 moles
E)26 moles

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
66
The figure shows a molecular-level diagram of reactant molecules for the reaction 2H2(g) + O2(g) 2H2O(l) 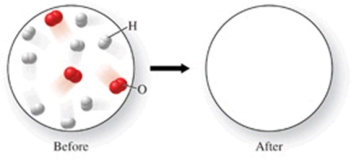 List the number and formulas of the molecules that should be present after the reaction takes place.
List the number and formulas of the molecules that should be present after the reaction takes place.
A)2H2O + 6H2 + 2O2
B)3H2O + 5H2 + O2
C)4H2O + 4H2 + O2
D)6H2O + 2H2 + O2
E)6H2O + 2H2
 List the number and formulas of the molecules that should be present after the reaction takes place.
List the number and formulas of the molecules that should be present after the reaction takes place.A)2H2O + 6H2 + 2O2
B)3H2O + 5H2 + O2
C)4H2O + 4H2 + O2
D)6H2O + 2H2 + O2
E)6H2O + 2H2

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
67
In the process of obtaining lead from PbS, or galena, the galena is "roasted" (heated in the presence of oxygen), so that the following reaction occurs: 2PbS(s) + 3O2(g) 2PbO(s) + 2SO2(g) If 35.2 g of PbS is mixed with 15.5 g of oxygen, how many grams of PbO should form?
A)32.8 g
B)72.1 g
C)105 g
D)65.7 g
E)49.2 g
A)32.8 g
B)72.1 g
C)105 g
D)65.7 g
E)49.2 g

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
68
Nitrogen monoxide reacts with oxygen according to the following reaction: 2NO(g) + O2(g) 2NO2(g) If 12 moles of nitrogen monoxide are combined with 10 moles of oxygen, how many moles of NO2 should form?
A)2 moles
B)5 moles
C)6 moles
D)10 moles
E)12 moles
A)2 moles
B)5 moles
C)6 moles
D)10 moles
E)12 moles

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
69
Nitrogen monoxide reacts with oxygen according to the following reaction: 2NO(g) + O2(g) 2NO2(g) If 10 moles of nitrogen monoxide are combined with 4 moles of oxygen, how many moles of NO2 should form?
A)4 moles
B)8 moles
C)10 moles
D)12 moles
E)14 moles
A)4 moles
B)8 moles
C)10 moles
D)12 moles
E)14 moles

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
70
Iron metal reacts with chlorine gas according to the following equation: 2Fe(s) + 3Cl2(g) 2FeCl3(s) If 25.6 g each of iron and chlorine are combined, how much FeCl3 should form?
A)74.3 g
B)113 g
C)39.0 g
D)49.5 g
E)26.0 g
A)74.3 g
B)113 g
C)39.0 g
D)49.5 g
E)26.0 g

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
71
Consider the reaction N2(g) + O2(g) 2NO(g).The molecular image represents a mixture of N2(g) and O2(g) just before reaction occurs.What is the limiting reactant, and how much of the excess reactant remains after the reaction is complete? The image contains 2 N2 molecules and 4 O2 molecules. 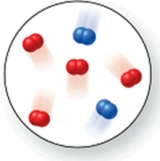
A)N2(g), 1 O2(g)
B)N2(g), 2 O2(g)
C)N2(g), 3 O2(g)
D)O2(g), 1 N2(g)
E)N2(g), 0 O2(g)

A)N2(g), 1 O2(g)
B)N2(g), 2 O2(g)
C)N2(g), 3 O2(g)
D)O2(g), 1 N2(g)
E)N2(g), 0 O2(g)

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
72
Consider the following reaction: 3NO2(g) + H2O(l) 2HNO3(l) + NO(g) How many moles of the excess reactant remain if 4.00 moles of H2O and 10.00 moles of NO2 are mixed?
A)0.67 mol H2O
B)2.00 mol NO2
C)3.33 mol H2O
D)6.00 mol NO2
E)8.00 mol NO2
A)0.67 mol H2O
B)2.00 mol NO2
C)3.33 mol H2O
D)6.00 mol NO2
E)8.00 mol NO2

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
73
Iron metal reacts with hydrochloric acid as follows: 2Fe(s) + 6HCl(aq) 2FeCl3(aq) + 3H2(g) If 35.6 g of iron react with excess HCl, and 98.6 g of FeCl3 are collected, what is the percent yield of FeCl3?
A)103%
B)104%
C)95.7%
D)63.0%
E)not enough information given
A)103%
B)104%
C)95.7%
D)63.0%
E)not enough information given

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
74
Iron metal reacts with hydrochloric acid as follows: 2Fe(s) + 6HCl(aq) 2FeCl3(aq) + 3H2(g) If 22.4 g of iron react with excess HCl, and 59.4 g of FeCl3 are collected, what is the percent yield of Al2(SO4)3?
A)65.0%
B)109%
C)91.4%
D)73.0%
E)not enough information given
A)65.0%
B)109%
C)91.4%
D)73.0%
E)not enough information given

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
75
Iron metal reacts with chlorine gas according to the following equation: 2Fe(s) + 3Cl2(g) 2FeCl3(s) If 35.0 g each of iron and chlorine are combined, how much FeCl3 should form?
A)102 g
B)155 g
C)53.4 g
D)80.0 g
E)68.0 g
A)102 g
B)155 g
C)53.4 g
D)80.0 g
E)68.0 g

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
76
What mass (in grams) of SF6 should be produced by the following reaction if 7.00 g of sulfur is mixed with 9.00 g of fluorine? S + 3F2 SF6
A)24.0
B)6.40
C)11.1
D)32.0
E)16.0
A)24.0
B)6.40
C)11.1
D)32.0
E)16.0

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
77
Consider the reaction N2(g) + 2O2(g) 2NO2(g).The molecular image represents a mixture of N2(g) and O2(g) just before reaction occurs.What is the limiting reactant, and how much of the excess reactant remains after the reaction is complete? The image contains 3 N2 molecules and 9 O2 molecules. 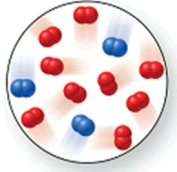
A)N2(g), 6 O2(g)
B)O2(g), 1 N2(g)
C)N2(g), 3 O2(g)
D)O2(g), 2 N2(g)
E)N2(g), 7 O2(g)

A)N2(g), 6 O2(g)
B)O2(g), 1 N2(g)
C)N2(g), 3 O2(g)
D)O2(g), 2 N2(g)
E)N2(g), 7 O2(g)

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
78
Aluminum reacts with oxygen according to the following reaction: 4Al(s) + 3O2(g) 2Al2O3(s) If 12 moles of aluminum are combined with 6 moles of oxygen, how many moles of Al2O3 should form?
A)4 moles
B)8 moles
C)6 moles
D)12 moles
E)20 moles
A)4 moles
B)8 moles
C)6 moles
D)12 moles
E)20 moles

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
79
If the theoretical yield for a reaction is 54.9 g, and 51.3 g of product are actually obtained, the percent yield is:
A)0.934%
B)93.4%
C)107%
D)3.60%
E)not enough information given
A)0.934%
B)93.4%
C)107%
D)3.60%
E)not enough information given

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
80
In the process of obtaining lead from PbS, or galena, the galena is "roasted" (heated in the presence of oxygen), so that the following reaction occurs: 2PbS(s) + 3O2(g) 2PbO(s) + 2SO2(g) If 50.0 g of PbS are mixed with 25.0 g of oxygen, how many grams of PbO should form?
A)116 g
B)46.6 g
C)163 g
D)69.9 g
E)93.2 g
A)116 g
B)46.6 g
C)163 g
D)69.9 g
E)93.2 g

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck



