Deck 4: Stereochemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/68
Play
Full screen (f)
Deck 4: Stereochemistry
1
Sertraline (shown below) is the active ingredient in the antidepressant Zoloft.
![<strong>Sertraline (shown below) is the active ingredient in the antidepressant Zoloft. Pure sertraline has a specific rotation [α] of +37.9° in methanol solution. What would the observed rotation α be for a mixture of 5.75g of pure sertraline in 30.0 ml of methanol if the solution is placed in a 10.0 cm polarimeter cell?</strong> A) 72.6° B) 19.8° C) 7.26° D) 198° E) 0.726°](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7c34_bbb7_2c73_b10d_85e37ac2028c_TB34225555_11.jpg)
Pure sertraline has a specific rotation [α] of +37.9° in methanol solution. What would the observed rotation α be for a mixture of 5.75g of pure sertraline in 30.0 ml of methanol if the solution is placed in a 10.0 cm polarimeter cell?
A) 72.6°
B) 19.8°
C) 7.26°
D) 198°
E) 0.726°
![<strong>Sertraline (shown below) is the active ingredient in the antidepressant Zoloft. Pure sertraline has a specific rotation [α] of +37.9° in methanol solution. What would the observed rotation α be for a mixture of 5.75g of pure sertraline in 30.0 ml of methanol if the solution is placed in a 10.0 cm polarimeter cell?</strong> A) 72.6° B) 19.8° C) 7.26° D) 198° E) 0.726°](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7c34_bbb7_2c73_b10d_85e37ac2028c_TB34225555_11.jpg)
Pure sertraline has a specific rotation [α] of +37.9° in methanol solution. What would the observed rotation α be for a mixture of 5.75g of pure sertraline in 30.0 ml of methanol if the solution is placed in a 10.0 cm polarimeter cell?
A) 72.6°
B) 19.8°
C) 7.26°
D) 198°
E) 0.726°
7.26°
2
The absolute configuration of trans,trans-3,4-dibromo-5-methyl-1-cyclohexene below is: 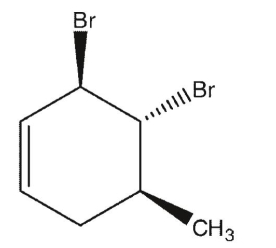
A) 3 R, 4R, 5R
B) 3S, 4S, 5S
C) 3R, 4S, 5S
D) 3 R, 4R, 5S
E) 3S, 4S, 5R

A) 3 R, 4R, 5R
B) 3S, 4S, 5S
C) 3R, 4S, 5S
D) 3 R, 4R, 5S
E) 3S, 4S, 5R
3 R, 4R, 5S
3
Which of the following pairs of molecules are enantiomers?
A)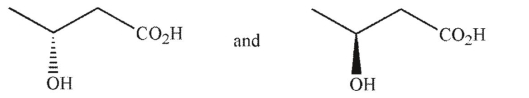
B)
C)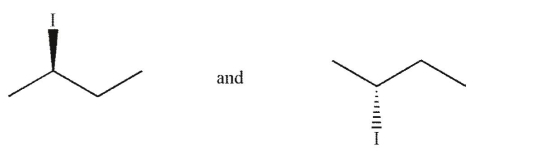
D)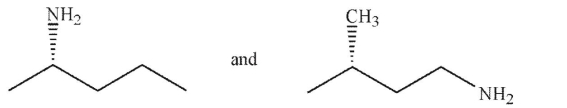
E)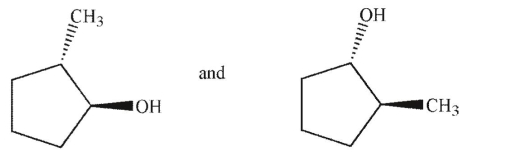
A)
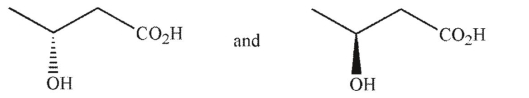
B)

C)

D)

E)

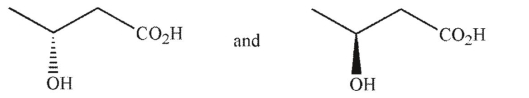
4
The different odors of (R)-(-) -carvone and (S) -(+) -carvone are best explained as being due to:
A) Differences in volatility between the two compounds
B) Differences in how the two compounds interact with chiral nasal receptors
C) Differences in the origins of the compounds: the (R)-compound is from spearmint, while the (S)-compound is from caraway
D) Differences in water solubility of the two compounds
E) Differences in how the body digests the two compounds
A) Differences in volatility between the two compounds
B) Differences in how the two compounds interact with chiral nasal receptors
C) Differences in the origins of the compounds: the (R)-compound is from spearmint, while the (S)-compound is from caraway
D) Differences in water solubility of the two compounds
E) Differences in how the body digests the two compounds

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following phrases correctly describes a racemic mixture?
A)equal mixture of enantiomers
B)unequal mixture of enantiomers
C)equal mixture of diastereomers
D)unequal mixture of diastereomers
E)optically active
A)equal mixture of enantiomers
B)unequal mixture of enantiomers
C)equal mixture of diastereomers
D)unequal mixture of diastereomers
E)optically active

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
6
How many stereogenic carbons are in trans-2,3-dideuterobicyclo[2.2.1]heptane?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following structures is achiral?
A)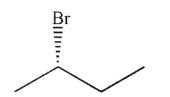
B)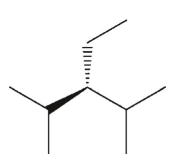
C)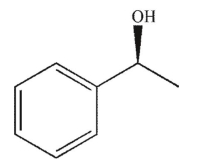
D)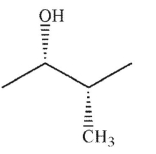
E)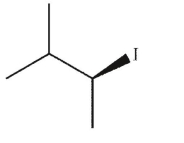
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
8
Which of these pairs of compounds would be classified as stereoisomers?
A) cis-2-hexene and trans-2-hexene
B) cis-2-hexene and trans-3-hexene
C) cis-2-hexene and cis-3-hexene
D) cis-1,2-dimethylcyclobutane and c i s-1,3 -dimethylcyclobutane
E) 2,2 -dimethylbutane and 2,3-dimethylbutane
A) cis-2-hexene and trans-2-hexene
B) cis-2-hexene and trans-3-hexene
C) cis-2-hexene and cis-3-hexene
D) cis-1,2-dimethylcyclobutane and c i s-1,3 -dimethylcyclobutane
E) 2,2 -dimethylbutane and 2,3-dimethylbutane

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following molecules is achiral?
A)trans-1,2-dimethylcyclobutane
B)cis-3,4-dimethyl-3-hexene
C)2,3-dimethylpentane
D)4-methyl-2-hexyne
E)3-methylcyclopentene
A)trans-1,2-dimethylcyclobutane
B)cis-3,4-dimethyl-3-hexene
C)2,3-dimethylpentane
D)4-methyl-2-hexyne
E)3-methylcyclopentene

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following pairs of molecules are related as diastereomers?
A)
B)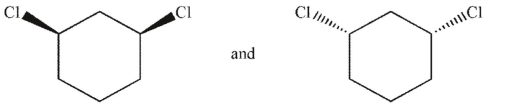
C)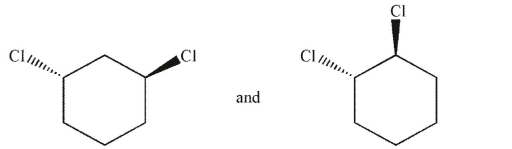
D)
E)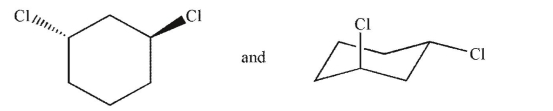
A)

B)
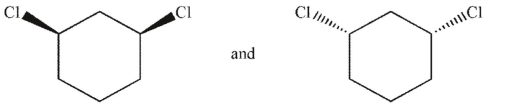
C)

D)

E)
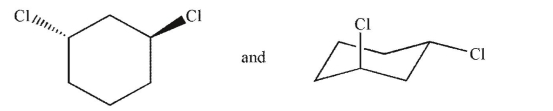

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following structures is chiral?
A)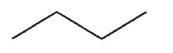
B)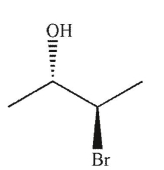
C)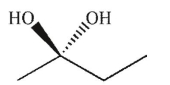
D)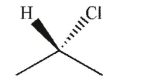
E)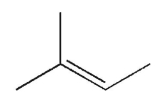
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following statements is true about the rotation of plane-polarized light by chiral compounds?
A)There is a direct connection between R/S configuration and the direction of rotation.
B)A mixture of enantiomers will have a larger optical rotation than either pure enantiomer.
C)Plane-polarized light is produced by passing light through an electromagnetic field.
D)The rotation of plane-polarized light is a physical property of chiral compounds.
E)The enantiomer that rotates plane-polarized light clockwise is called levorotatory, or l.
A)There is a direct connection between R/S configuration and the direction of rotation.
B)A mixture of enantiomers will have a larger optical rotation than either pure enantiomer.
C)Plane-polarized light is produced by passing light through an electromagnetic field.
D)The rotation of plane-polarized light is a physical property of chiral compounds.
E)The enantiomer that rotates plane-polarized light clockwise is called levorotatory, or l.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following statements is true about enantiomers?
A)Enantiomers must have at least one stereogenic atom.
B)An enantiomer has a nonsuperimposable mirror image.
C)Enantiomers have identical physical properties.
D)Enantiomers have identical chemical properties.
E)A mixture of two enantiomers will have the same properties as the two separate enantiomers.
A)Enantiomers must have at least one stereogenic atom.
B)An enantiomer has a nonsuperimposable mirror image.
C)Enantiomers have identical physical properties.
D)Enantiomers have identical chemical properties.
E)A mixture of two enantiomers will have the same properties as the two separate enantiomers.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following molecules contains a stereogenic carbon properly designated as R?
A)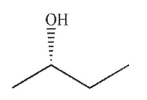
B)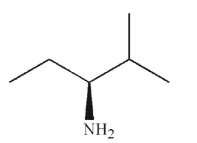
C)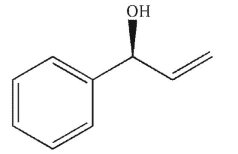
D)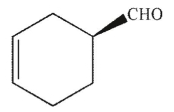
E)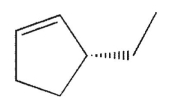
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following pairs of molecules are not enantiomers?
A)
B)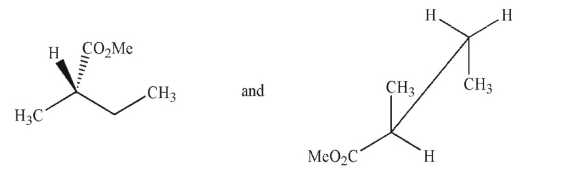
C)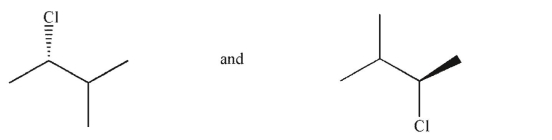
D)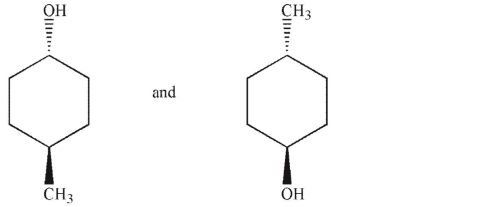
E)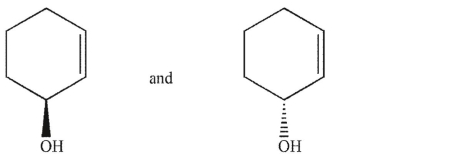
A)

B)

C)
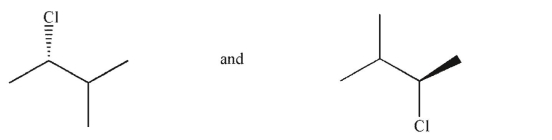
D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following structures is the enantiomer of the molecule shown here? 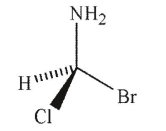
A)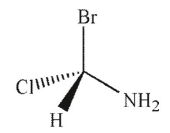
B)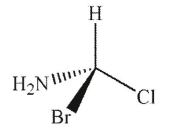
C)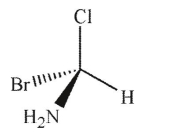
D)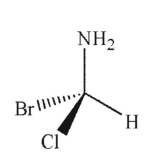
E)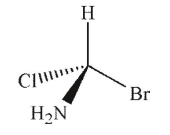

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
17
If a pure sample of molecule X has an optical rotation of +15.8 degrees, what is the optical rotation of a pure sample of the enantiomer of molecule X?
A) +15.8
B) -15.8
C) 0
D) +164.2
E) This value can only be determined experimentally.
A) +15.8
B) -15.8
C) 0
D) +164.2
E) This value can only be determined experimentally.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following molecules contains a stereogenic center properly designated as S?
A)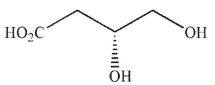
B)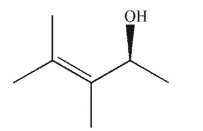
C)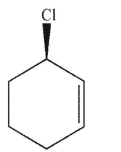
D)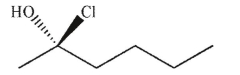
E)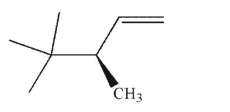
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following molecules could be chiral?
A)2-methylhexane
B)3-methylpentane
C)2,4,6,8-tetramethylnonane
D)2,3,4-trimethylpentane
E)3-ethyl-2-methylpentane
A)2-methylhexane
B)3-methylpentane
C)2,4,6,8-tetramethylnonane
D)2,3,4-trimethylpentane
E)3-ethyl-2-methylpentane

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following structures is chiral?
A)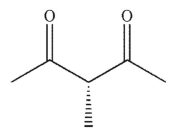
B)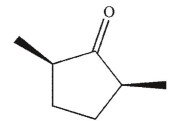
C)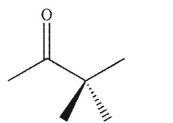
D)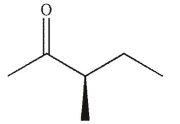
E)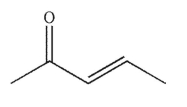
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
21
Which term best describes the relationship between the molecules shown? 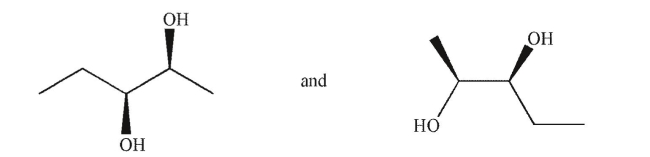
A)conformational isomers
B)diastereomers
C)enantiomers
D)identical
E)structural isomers
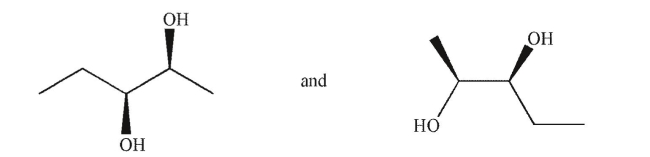
A)conformational isomers
B)diastereomers
C)enantiomers
D)identical
E)structural isomers

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
22
Which of these compounds is chiral? 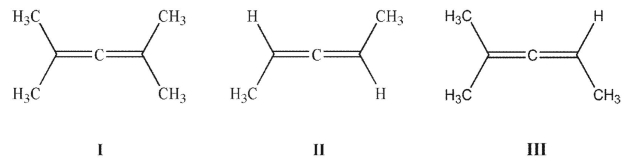
A) I
B) II
C) III
D) I and II
E) I and III

A) I
B) II
C) III
D) I and II
E) I and III

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
23
Which term best describes the relationship between the molecules shown? 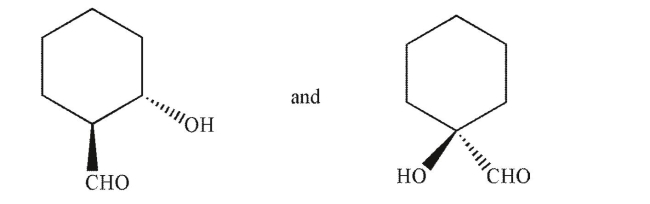
A)conformational isomers
B)diastereomers
C)enantiomers
D)identical
E)structural isomers

A)conformational isomers
B)diastereomers
C)enantiomers
D)identical
E)structural isomers

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following pairs of molecules are related as diastereomers?
A)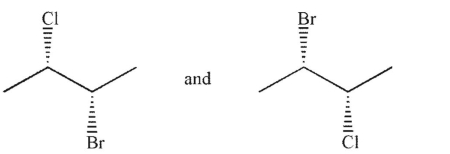
B)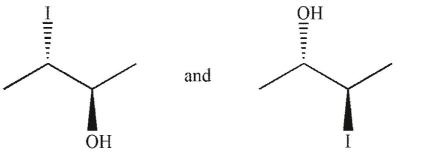
C)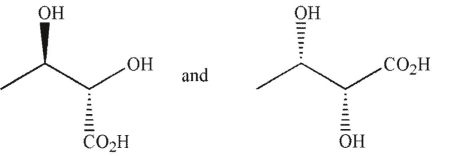
D)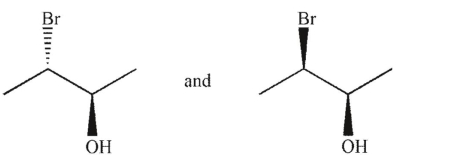
E)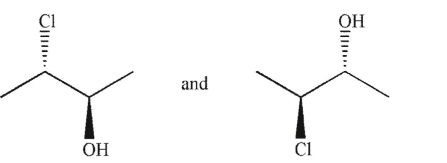
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following dichlorocycloalkanes is a meso compound?
A)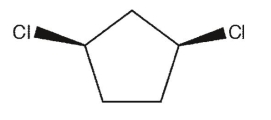
B)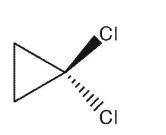
C)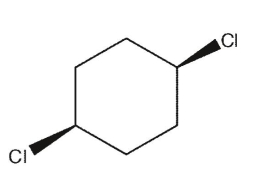
D)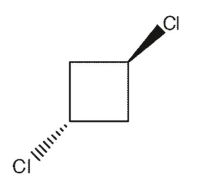
E)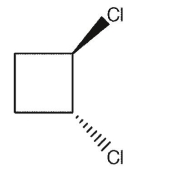
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following dichlorocycloalkanes is chiral?
A)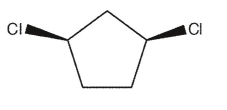
B)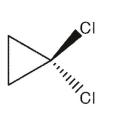
C)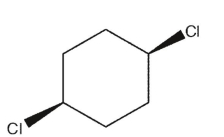
D)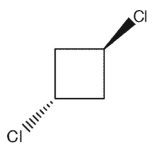
E)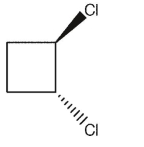
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
27
Assign the absolute configuration at the stereogenic center of the molecule shown here. 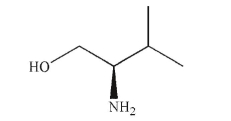


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
28
Draw the enantiomer of the molecule shown here. 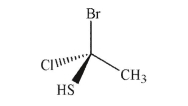


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following techniques could not be used to resolve a racemic mixture of organic acids?
A)React the mixture with brucine and separate the salts.
B)Crystallize the acids and manually separate the crystals.
C)React the acids with ammonia and separate the salts.
D)Elute a solution of the acids through a column containing a chiral stationary phase.
E)React the mixture using an enzyme as a catalyst.
A)React the mixture with brucine and separate the salts.
B)Crystallize the acids and manually separate the crystals.
C)React the acids with ammonia and separate the salts.
D)Elute a solution of the acids through a column containing a chiral stationary phase.
E)React the mixture using an enzyme as a catalyst.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
30
Which statement about diastereomers is always true?
A)A mixture containing a pair of diastereomers in equal amounts will be optically inactive.
B)If a molecule can be described as a diastereomer of another molecule, it is chiral.
C)All diastereomers are achiral.
D)Every chiral molecule has at least one diastereomer.
E)A pair of diastereomers are not mirror images of one another.
A)A mixture containing a pair of diastereomers in equal amounts will be optically inactive.
B)If a molecule can be described as a diastereomer of another molecule, it is chiral.
C)All diastereomers are achiral.
D)Every chiral molecule has at least one diastereomer.
E)A pair of diastereomers are not mirror images of one another.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
31
Which term best describes the relationship between the pair of molecules shown? 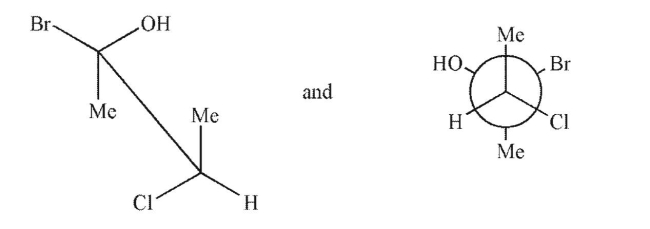
A)conformational isomers
B)diastereomers
C)enantiomers
D)identical
E)structural isomers

A)conformational isomers
B)diastereomers
C)enantiomers
D)identical
E)structural isomers

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
32
Which is not a stereoisomer of the molecule shown here? 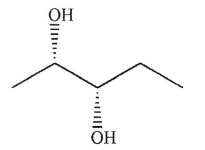
A)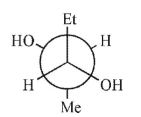
B)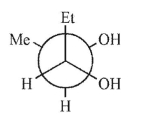
C)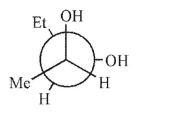
D)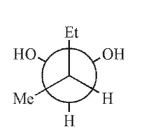
E)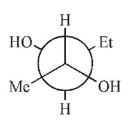

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
33
How many stereoisomers exist for this compound? 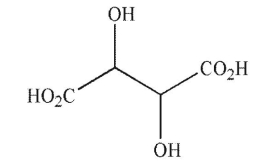
A) 1
B) 2
C) 3
D) 4
E) 5

A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
34
Assign the absolute configuration at the stereogenic center in the molecule shown here. 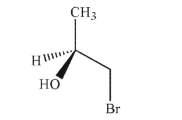


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following molecules is achiral and meso?
A)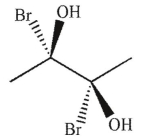
B)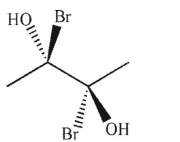
C)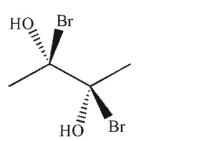
D)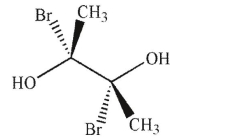
E)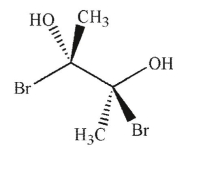
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
36
The relationship between the two molecules shown below is best described as 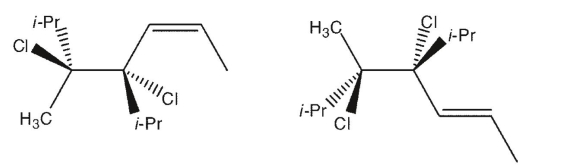
A) same compound
B) diastereomers
C) structural isomers
D) enantiomers
E) conformational isomers

A) same compound
B) diastereomers
C) structural isomers
D) enantiomers
E) conformational isomers

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following is a meso compound?
A)
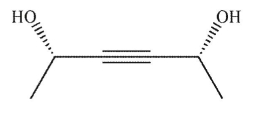
B)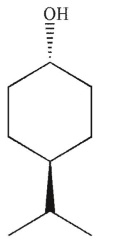
C)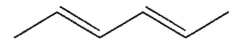
D)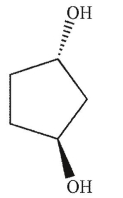
E)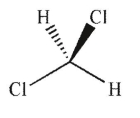
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
38
Put a star next to each stereogenic carbon in the structure shown here. 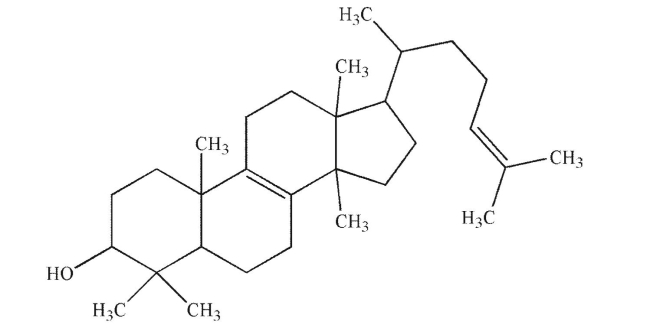


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following structures is the enantiomer of the structure shown here? 
A)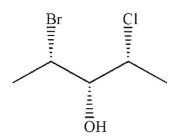
B)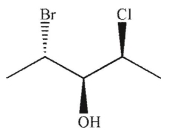
C)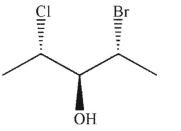
D)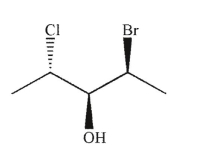
E)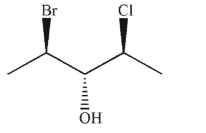

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
40
What is the correct term that describes the process for separating a pair of enantiomers?
A)Racemization
B)Resolution
C)Equilibration
D)Rotation
E)Reconfiguration
A)Racemization
B)Resolution
C)Equilibration
D)Rotation
E)Reconfiguration

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
41
Distinguish between the terms enantiomers and diastereomers.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
42
Assign the absolute configuration of each stereogenic center in the natural product shown here as R or S. 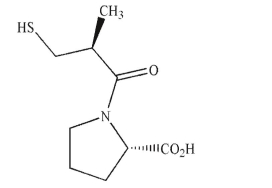


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
43
How many stereoisomers are possible for this molecule? 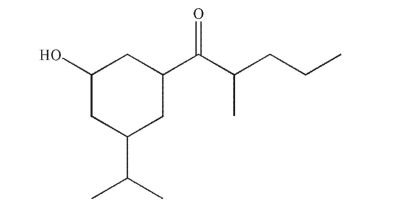


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
44
Explain why butane is an achiral molecule even though it has two enantiomeric conformations.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
45
Draw the R isomer of this compound. 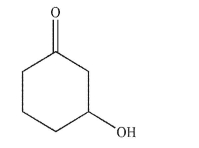


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
46
d-Sucrose is common table sugar.Its enantiomer, l-sucrose, has been investigated as a
nonnutritive sweetener.Explain why l-sucrose would function as a sweetener, but would not
generate the same number of dietician's calories as the d-enantiomer.
nonnutritive sweetener.Explain why l-sucrose would function as a sweetener, but would not
generate the same number of dietician's calories as the d-enantiomer.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
47
Sucrose has a specific rotation of +66.5° at 25° . A solution of an unknown pure substance in ethanol (c=4.62 g/10 mL) shows an observed rotation of +20.4°. Is it possible that the substance is sucrose? Recall that [α]=α/cl , where [α] is the specific rotation, α is the observed rotation, the path length, l , is 1 decimeter; and c is the concentration of the sample.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
48
Draw the S enantiomer of 3,3,4-trimethylhexane.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
49
Draw the two major products of the addition reaction below, showing stereochemistry. 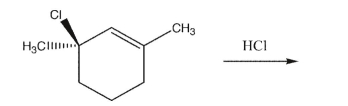 What is the stereochemical relationship between the two products? Are both products optically
What is the stereochemical relationship between the two products? Are both products optically
active?
 What is the stereochemical relationship between the two products? Are both products optically
What is the stereochemical relationship between the two products? Are both products opticallyactive?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
50
Define optical activity.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
51
Draw all stereoisomers of the molecule shown here. 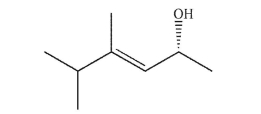


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
52
For each pair of molecules, identify whether the pairs are enantiomers, diastereomers, or the same
compound/conformers.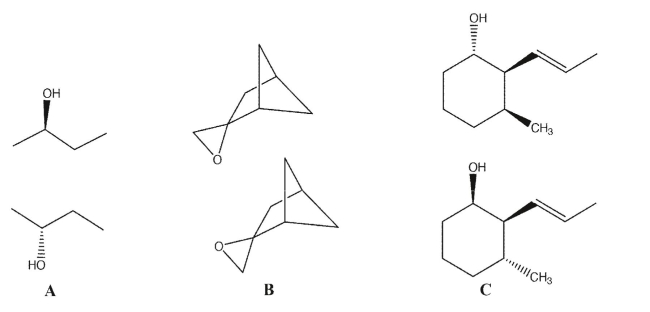
compound/conformers.


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
53
Draw a staggered Newman projection of R-2-pentanol looking down the C2-C3 bond.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
54
Label each of the following molecules as chiral, achiral, or achiral and meso. 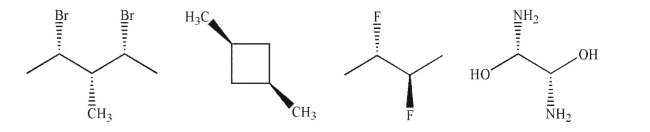


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
55
Assign the absolute configuration of the stereogenic center in the molecule shown here. 


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
56
Draw all diastereomers of the molecule shown here. 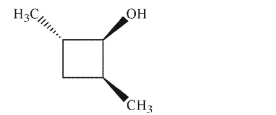


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
57
Draw the structure of (3R, 4S)-2,3,4 -trimethylheptane.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
58
Identify all stereogenic carbon atoms in the molecule shown here and assign the absolute
configuration of each.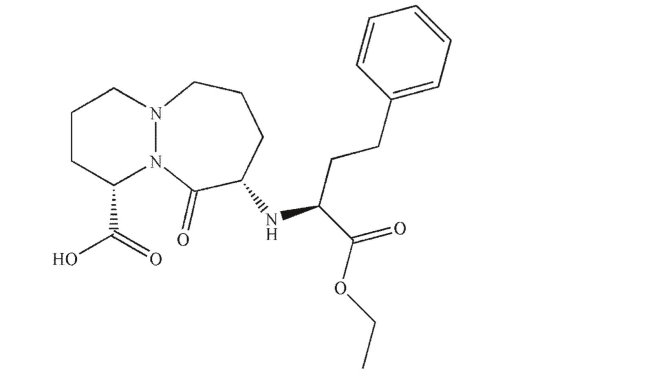
configuration of each.


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
59
What are the differences in the physical properties of a pair of enantiomers?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
60
Assign the absolute configuration of each stereogenic center in the natural product shown as R
or S.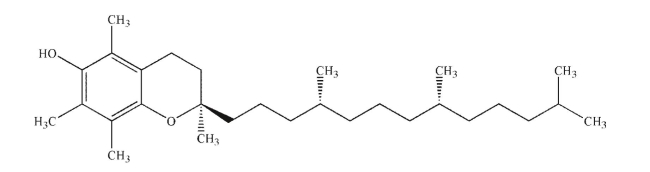
or S.


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
61
Draw all possible stereoisomers having the skeletal formula shown here.Show stereochemistry
using wedge and dash notation.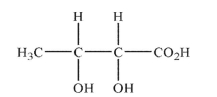
using wedge and dash notation.


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
62
For the following compounds determine if a) they have one or more stereogenic atoms and b)
they are chiral.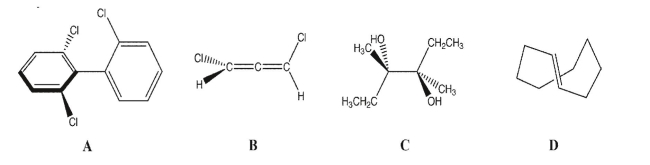
they are chiral.


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
63
Identify each pair of molecules as conformational isomers, diastereomers, enantiomers, identical,
or structural isomers.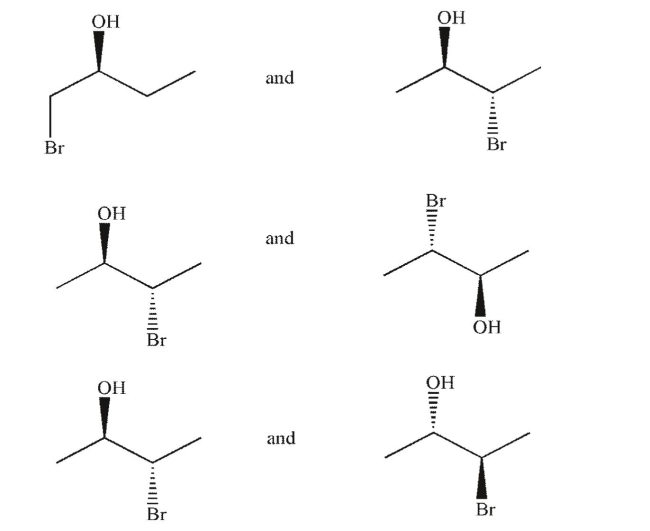
or structural isomers.


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
64
Dextromethorphan (D-meth) and its enantiomer, levomethorphan (L-meth), have very different
pharmacological effects.D-meth is the active ingredient in many cough suppressants, while
L-meth is an opioid analgesic.Explain how R-mandelic acid could be used to resolve a mixture of
these two drugs if they were produced as a racemic mixture.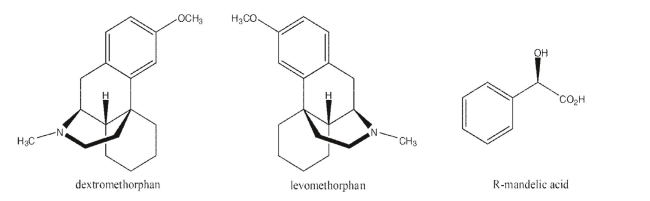
pharmacological effects.D-meth is the active ingredient in many cough suppressants, while
L-meth is an opioid analgesic.Explain how R-mandelic acid could be used to resolve a mixture of
these two drugs if they were produced as a racemic mixture.


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
65
Explain why compound A is chiral but compound B is not. 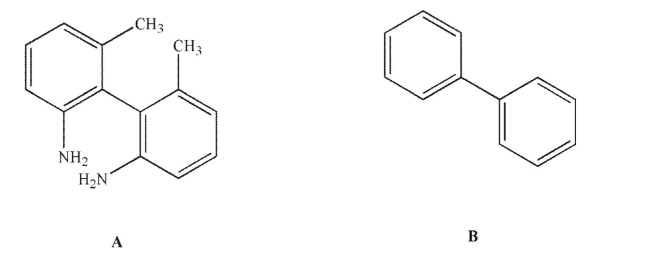


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
66
Draw all stereoisomers of the compound shown and label all stereogenic carbon atoms as R or S. 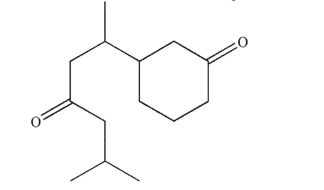


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
67
Identify each pair of molecules as conformational isomers, diastereomers, enantiomers, identical,
or structural isomers.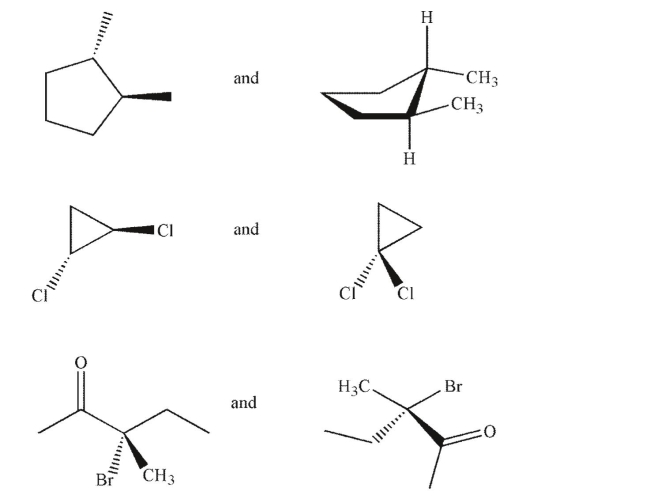
or structural isomers.


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
68
Draw all stereoisomers of the molecule shown here. 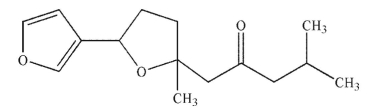


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck



