Deck 5: Rings
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/60
Play
Full screen (f)
Deck 5: Rings
1
Which of these cycloalkanes cannot relieve torsional strain?
A)cyclopropane
B)cyclobutane
C)cyclopentane
D)a and b
E)a and c
A)cyclopropane
B)cyclobutane
C)cyclopentane
D)a and b
E)a and c
cyclopropane
2
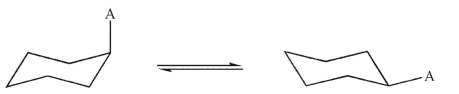
What is the relationship between the two structures shown here? 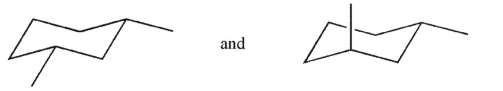
A) They are enantiomeric conformations.
B) They are identical.
C) They are enantiomers.
D) They are diastereomers.
E) They are constitutional isomers.
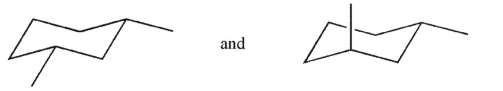
A) They are enantiomeric conformations.
B) They are identical.
C) They are enantiomers.
D) They are diastereomers.
E) They are constitutional isomers.
They are diastereomers.
3
Using the heats of combustion per CH2 given, select the least stable compound in the set.All values are in kcal/mol. 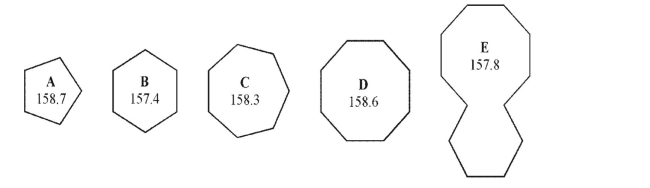
A)A
B)B
C)C
D)D
E)E

A)A
B)B
C)C
D)D
E)E
A
4
What is the highest-energy conformation of cyclohexane?
A)chair
B)full boat
C)twist boat
D)half chair
E)b and c are identical.
A)chair
B)full boat
C)twist boat
D)half chair
E)b and c are identical.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following molecules is chiral?
A) cis-1,2-dimethylcyclopropane
B) trans-1,3-dimethylcyclobutane
C) cis-1,3-dimethylcyclohexane
D) trans-1,4-dimethylcyclohexane
E) trans-1,3-dimethylcyclohexane
A) cis-1,2-dimethylcyclopropane
B) trans-1,3-dimethylcyclobutane
C) cis-1,3-dimethylcyclohexane
D) trans-1,4-dimethylcyclohexane
E) trans-1,3-dimethylcyclohexane

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following words describes a low-energy conformation of cyclopentane?
A)boat
B)chair
C)envelope
D)half boat
E)twist chair
A)boat
B)chair
C)envelope
D)half boat
E)twist chair

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
7
How many signals would appear in the 13C NMR spectrum for this molecule?
A) 2
B) 3
C) 4
D) 5
E) 7

A) 2
B) 3
C) 4
D) 5
E) 7

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
8
Assuming 98.85 % of the molecules are in the conformation with the group A equatorial, what is the value of ΔG° for this equilibrium at 25°C? R=1.987 x10-3 kcal/mol K
A) -2.64 kcal/mol
B) -2.72 kcal/mol
C) 0.228 kcal/mol
D) 2.64 kcal/mol
E) 2.72 kcal/mol

A) -2.64 kcal/mol
B) -2.72 kcal/mol
C) 0.228 kcal/mol
D) 2.64 kcal/mol
E) 2.72 kcal/mol

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
9
Which double Newman projection matches the view of the molecule below along the C(1)-C(6) and C(3)-C(4) bonds? 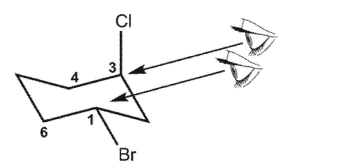
A)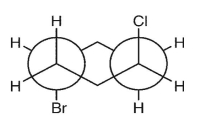
B)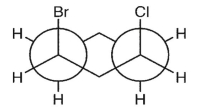
C)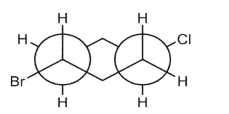
D)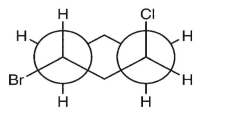
E)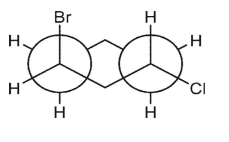

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
10
Which of these cycloalkanes is planar in its lowest energy conformation? 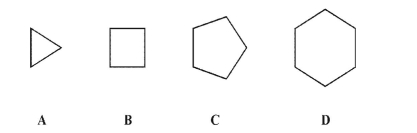
A)A
B)B
C)C
D)D
E)All of the above are planar in their lowest energy conformations.

A)A
B)B
C)C
D)D
E)All of the above are planar in their lowest energy conformations.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
11
What percentage of methylcyclohexane exists in the axial methyl conformation at equilibrium, given that Keq=19.5 at 25°C? R=1.987 x 10-3 kcal/mol K
A) 5 %
B) 19.5 %
C) 50 %
D) 95 %
E) 100 %
A) 5 %
B) 19.5 %
C) 50 %
D) 95 %
E) 100 %

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
12
For which of the following do you expect to have the greatest percentage of molecules in the conformation with the substituent in an equatorial position?
A)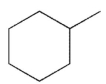
B)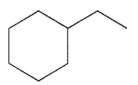
C)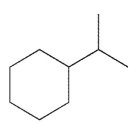
D)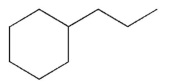
E)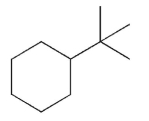
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
13
Consider the two chair conformations of trans-1,3-dimethylcyclohexane. Which of the following statements is/are true?
I. Keq=1
II. The molecule is chiral.
III. The two chair conformations are enantiomers.
A) I
B) I, II
C) I, III
D) I, II, III
E) II, III
I. Keq=1
II. The molecule is chiral.
III. The two chair conformations are enantiomers.
A) I
B) I, II
C) I, III
D) I, II, III
E) II, III

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
14
What type of strain does cyclobutane relieve by puckering?
A)angle
B)torsional
C)van der Waals
D)bond length
E)all of these
A)angle
B)torsional
C)van der Waals
D)bond length
E)all of these

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
15
Which of these structures represents the highest-energy conformation of cyclohexane?
A)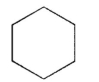
B)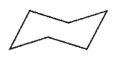
C)
D)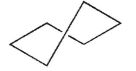
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following statements is true about this structure? 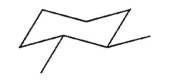
A) The two methyl groups are anti.
B) The two methyl groups are gauche.
C) The two methyl groups are eclipsed.
D) The conformation shown is the highest energy conformation for this molecule.
E) The molecule is achiral.

A) The two methyl groups are anti.
B) The two methyl groups are gauche.
C) The two methyl groups are eclipsed.
D) The conformation shown is the highest energy conformation for this molecule.
E) The molecule is achiral.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
17
The envelope and twist conformations of cyclopentane are lower energy than the planar one because they have
A)smaller bond angles.
B)larger bond angles.
C)fewer eclipsing interactions.
D)more flexibility.
E)no bent bonds.
A)smaller bond angles.
B)larger bond angles.
C)fewer eclipsing interactions.
D)more flexibility.
E)no bent bonds.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
18
Use the heat of combustion provided to select the most stable compound.All values are in kcal/mole.
A)Cyclooctane, 1269.2
B)Cyclononane, 1429.6
C)Cyclodecane, 1586.8
D)Cycloundecane, 1743.1
E)Cyclobutane, 499.8
A)Cyclooctane, 1269.2
B)Cyclononane, 1429.6
C)Cyclodecane, 1586.8
D)Cycloundecane, 1743.1
E)Cyclobutane, 499.8

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
19
Which of these compounds can adopt the most stable conformation?
A)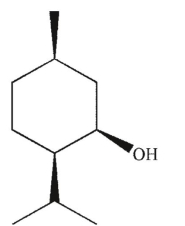
B)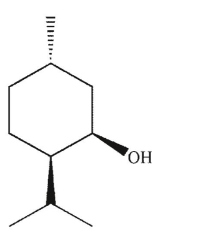
C)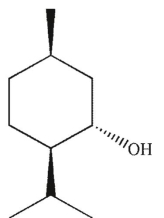
D)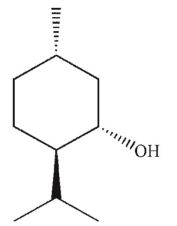
E)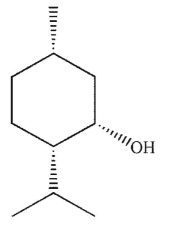
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
20
After ring flip, what type of position will substituent X occupy? 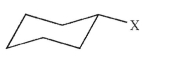
A)axial, up
B)axial, down
C)equatorial, up
D)equatorial, down
E)It depends on the nature of X.

A)axial, up
B)axial, down
C)equatorial, up
D)equatorial, down
E)It depends on the nature of X.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
21
Explain why the chair conformation of cyclohexane is considered to be strain free.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
22
Select any and all words that correctly describe the relationship between groups A and B in the structure shown here. 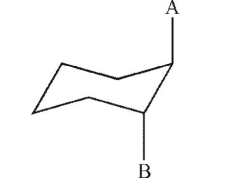
I. anti
II. gauche
III. eclipsed
IV. cis
V. trans
A) I
B) II
C) III
D) I, IV
E) I, V

I. anti
II. gauche
III. eclipsed
IV. cis
V. trans
A) I
B) II
C) III
D) I, IV
E) I, V

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
23
Which structure is fused?
A)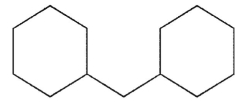
B)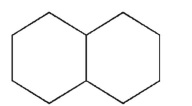
C)
D)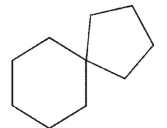
E)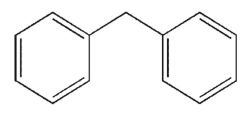
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
24
Draw the boat conformation of cyclohexane and identify the hydrogens that are the source of van
der Waals strain.
der Waals strain.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
25
Which three-dimensional structure is not one of the chair flips of the given molecule?
A)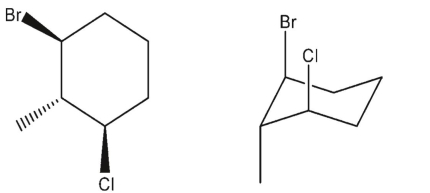
B)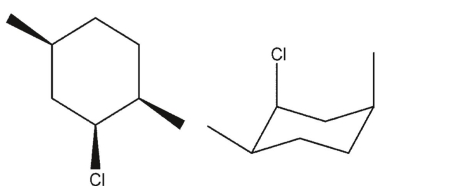
C)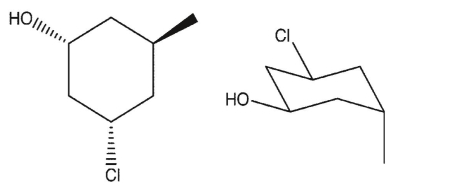
D)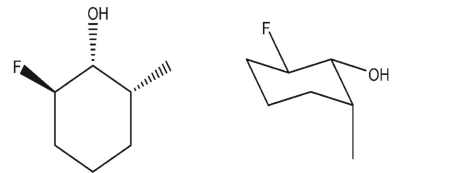
E)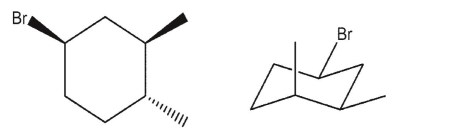
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
26
Select any and all words that correctly describe the relationship between groups A and B in the structure shown here. 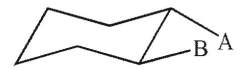
I. anti
II. gauche
III. eclipsed
IV. cis
V. trans
A) I
B) II
C) III
D) I and IV
E) II and V

I. anti
II. gauche
III. eclipsed
IV. cis
V. trans
A) I
B) II
C) III
D) I and IV
E) II and V

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
27
Which pair are not chair flips of each other?
A)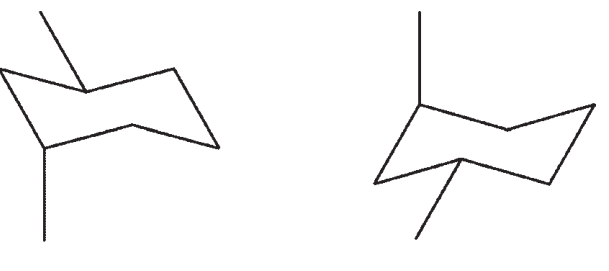
B)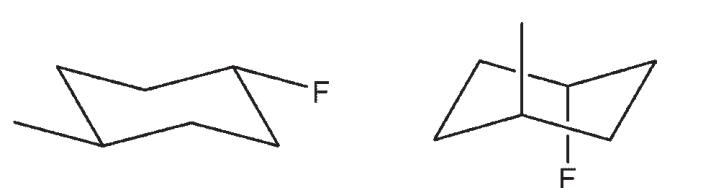
C)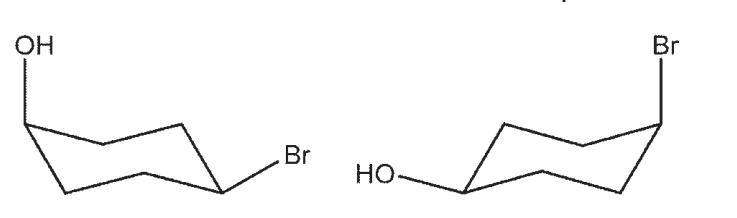
D)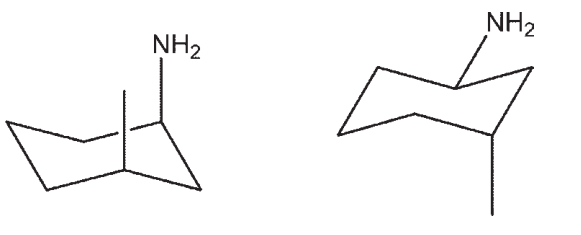
E)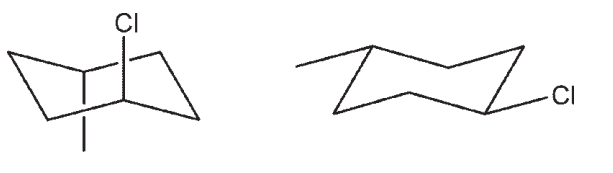
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
28
What is the correct structure for bicyclo[3.3.1]nonane?
A)![<strong>What is the correct structure for bicyclo[3.3.1]nonane? </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7c3f_4450_c2fe_b10d_8bf9877fdfce_TB34225555_11.jpg)
B)![<strong>What is the correct structure for bicyclo[3.3.1]nonane? </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7c3f_4452_e5df_b10d_49a7c9d07cd0_TB34225555_11.jpg)
C)![<strong>What is the correct structure for bicyclo[3.3.1]nonane? </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7c3f_4454_1e60_b10d_4780abe61693_TB34225555_11.jpg)
D)![<strong>What is the correct structure for bicyclo[3.3.1]nonane? </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7c3f_4455_56e1_b10d_27a46e35f523_TB34225555_11.jpg)
E)![<strong>What is the correct structure for bicyclo[3.3.1]nonane? </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7c3f_4456_8f62_b10d_4fee097420d4_TB34225555_11.jpg)
A)
![<strong>What is the correct structure for bicyclo[3.3.1]nonane? </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7c3f_4450_c2fe_b10d_8bf9877fdfce_TB34225555_11.jpg)
B)
![<strong>What is the correct structure for bicyclo[3.3.1]nonane? </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7c3f_4452_e5df_b10d_49a7c9d07cd0_TB34225555_11.jpg)
C)
![<strong>What is the correct structure for bicyclo[3.3.1]nonane? </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7c3f_4454_1e60_b10d_4780abe61693_TB34225555_11.jpg)
D)
![<strong>What is the correct structure for bicyclo[3.3.1]nonane? </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7c3f_4455_56e1_b10d_27a46e35f523_TB34225555_11.jpg)
E)
![<strong>What is the correct structure for bicyclo[3.3.1]nonane? </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7c3f_4456_8f62_b10d_4fee097420d4_TB34225555_11.jpg)

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the structures shown here is a spiro ring system?
A)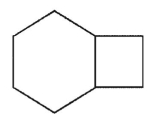
B)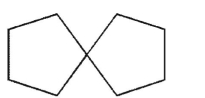
C)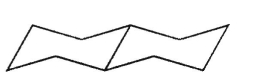
D)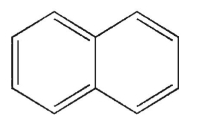
E)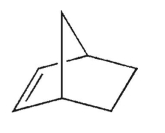
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following statements is/are true about the equilibrium shown here? 
I. Each structure is chiral.
II. Each structure is achiral.
III. Structure A is favored at equilibrium.
IV. Structure B is favored at equilibrium.
V. A and B are enantiomers.
VI. A and B are diastereomers.
A) I, III
B) I, III, VI
C) II, III, VI
D) I, III, V
E) I, IV

I. Each structure is chiral.
II. Each structure is achiral.
III. Structure A is favored at equilibrium.
IV. Structure B is favored at equilibrium.
V. A and B are enantiomers.
VI. A and B are diastereomers.
A) I, III
B) I, III, VI
C) II, III, VI
D) I, III, V
E) I, IV

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
31
Draw an energy versus conformation diagram for cyclohexane, showing all structures and their
relative energy levels.
relative energy levels.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
32
Which equilibrium constant must be precisely equal to 1?
A)
B)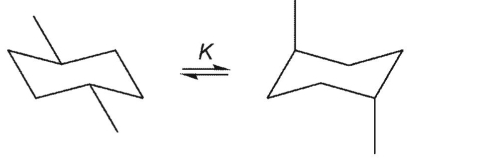
C)
D)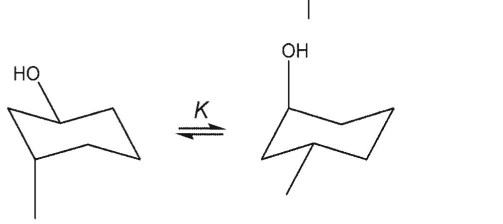
E)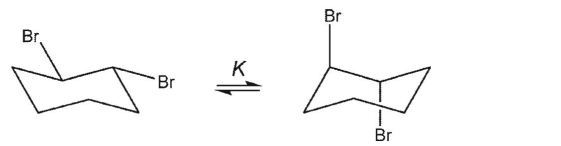
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
33
Draw the chair conformation of cyclohexane and identify all axial and equatorial hydrogens.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
34
What is the correct name for the structure shown here? ![<strong>What is the correct name for the structure shown here? </strong> A) Bicyclopentylcyclohexane B) Bicyclo[6.2]nonane C) Bicyclo[6.2.1]undecane D) Bicyclo[1.2] cyclononane E) Bicyclo[9.5]undecane](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7c3f_0070_d0cd_b10d_87868a3a2f34_TB34225555_11.jpg)
A) Bicyclopentylcyclohexane
B) Bicyclo[6.2]nonane
C) Bicyclo[6.2.1]undecane
D) Bicyclo[1.2] cyclononane
E) Bicyclo[9.5]undecane
![<strong>What is the correct name for the structure shown here? </strong> A) Bicyclopentylcyclohexane B) Bicyclo[6.2]nonane C) Bicyclo[6.2.1]undecane D) Bicyclo[1.2] cyclononane E) Bicyclo[9.5]undecane](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7c3f_0070_d0cd_b10d_87868a3a2f34_TB34225555_11.jpg)
A) Bicyclopentylcyclohexane
B) Bicyclo[6.2]nonane
C) Bicyclo[6.2.1]undecane
D) Bicyclo[1.2] cyclononane
E) Bicyclo[9.5]undecane

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
35
How many signals will appear in the 13C NMR spectrum for the molecule shown here?
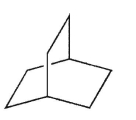
A) 2
B) 3
C) 4
D) 6
E) 8

A) 2
B) 3
C) 4
D) 6
E) 8

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
36
Select any and all words that correctly describe the relationship between groups A and B in the structure shown here. 
I. anti
II. gauche
III. eclipsed
IV. cis
V. trans
A) I
B) II
C) III
D) I and V
E) II and V

I. anti
II. gauche
III. eclipsed
IV. cis
V. trans
A) I
B) II
C) III
D) I and V
E) II and V

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following statements is true?
A) trans-Decalin cannot undergo ring flip.
B) cis-Decalin cannot undergo ring flip.
C) In trans-decalin, the bridgehead hydrogens are both equatorial.
D) In cis-decalin, the bridgehead hydrogens are both axial.
E) Both a and c.
A) trans-Decalin cannot undergo ring flip.
B) cis-Decalin cannot undergo ring flip.
C) In trans-decalin, the bridgehead hydrogens are both equatorial.
D) In cis-decalin, the bridgehead hydrogens are both axial.
E) Both a and c.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
38
Draw a Newman projection of cyclohexane looking along two C-C bonds that are separated by one carbon.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following structures is trans-decalin?
A)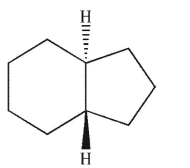
B)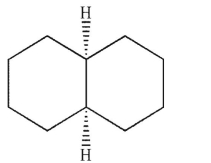
C)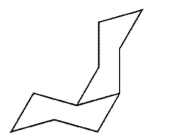
D)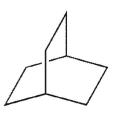
E)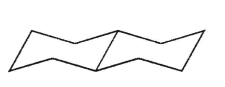
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
40
Draw the envelope conformation of cyclopentane, showing all hydrogens.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
41
Draw a bond line structure, showing stereochemistry with wedge-and-dash notation, for 1R, 2S, 4R-4-chloro- 1,2 -dimethylcyclohexane.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
42
Explain why axial methylcyclohexane and equatorial methylcyclohexane cannot be separated from
each other at room temperature.
each other at room temperature.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
43
Draw both chair forms of cis-1,3-dimethylcyclohexane and identify all sources of strain in the less
stable chair conformation.
stable chair conformation.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
44
Provide the systematic IUPAC name for the structure shown here. 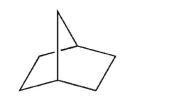


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
45
Draw the ring flip of the structure shown here. 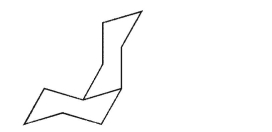


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
46
Explain why the conformer of 5 -methyl-1,3-dixoane with the methyl group axial exists in a higher percentage at equilibrium than the corresponding one for methylcyclohexane. That is, explain why K2>KI below.
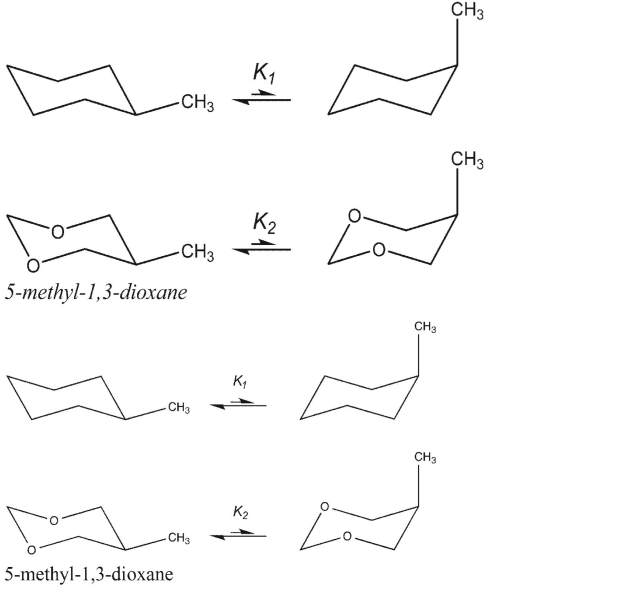


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
47
Draw both chair conformations of trans-1-isopropyl-2-methylcyclohexane and identify the more
stable chair conformation.
stable chair conformation.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
48
Use Newman projections of the less stable conformer of methylcyclohexane to identify all
interactions that raise its energy relative to the more stable conformer.
interactions that raise its energy relative to the more stable conformer.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
49
Draw the two chair conformations of methylcyclohexane.Identify the more stable conformation
and identify the source(s) of strain in the less stable chair conformation.
and identify the source(s) of strain in the less stable chair conformation.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
50
Draw the most stable chair conformation of 1 S, 2 R-1 -tert -butyl-2-methylcyclohexane.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
51
Provide the systematic IUPAC name for the structure shown here. 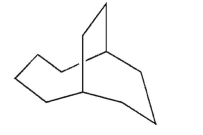


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
52
Draw the structure of bicyclo[3.2.1]octane.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
53
Which molecule do you expect to be more stable, A or B? 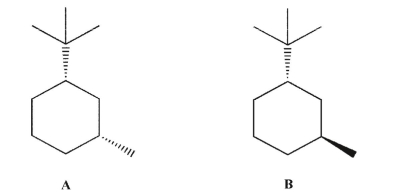


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
54
Draw a Newman projection along the indicated bond of the conformer below. 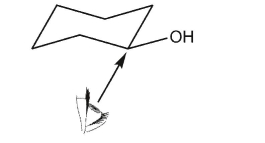


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
55
Draw the most stable conformer of the molecule below in three dimensions. 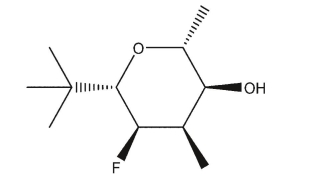


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
56
Draw the most stable chair conformation of this sugar. 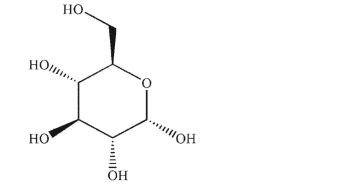


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
57
Draw the most stable conformer of trans-1-bromo-4-methylcyclohexane in three dimensions.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
58
Convert the chair structure shown to a flat bond-line drawing, using wedge-and-dash notation to
show stereochemistry.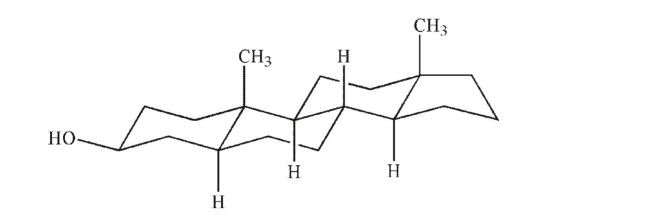
show stereochemistry.


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
59
Provide a name for the structure shown here, including R and S designation of stereogenic
carbons.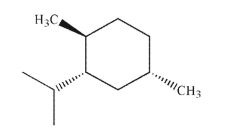
carbons.


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
60
Draw the most stable conformer of the molecule below in three dimensions. 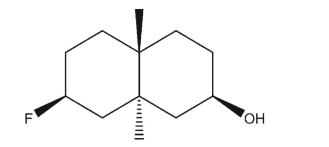


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck



