Deck 2: Alkanes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/65
Play
Full screen (f)
Deck 2: Alkanes
1
Which structure contains a hybrid orbital with a higher %s character than the hybrids found in any of the others?
A)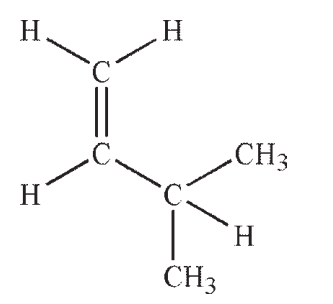
B)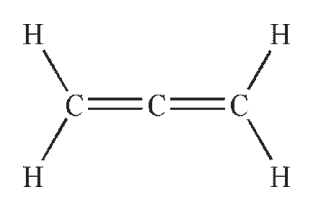
C)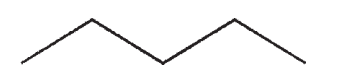
D)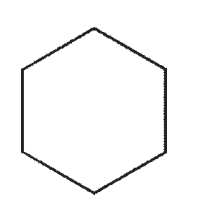
E)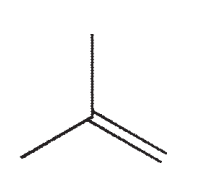
A)

B)

C)

D)

E)


2
Which of the following compounds are pairs of constitutional isomers?
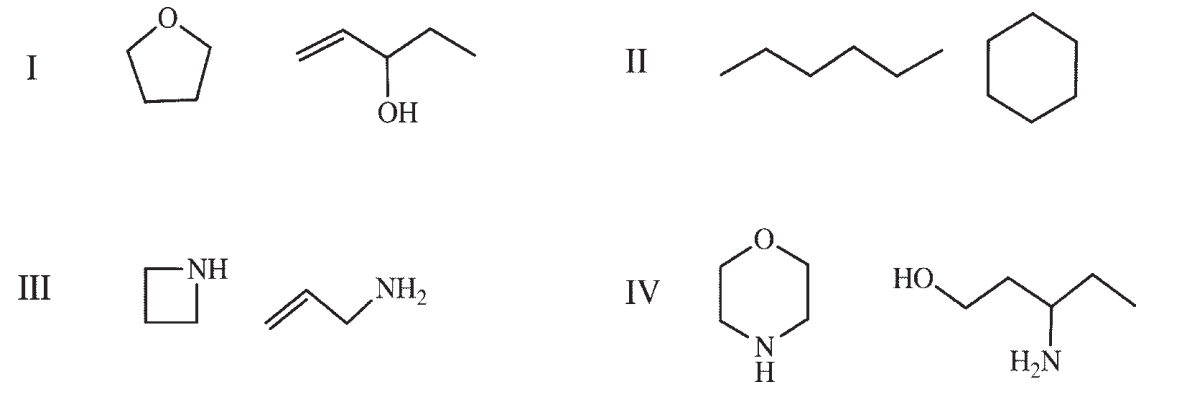
A) I and IV
B) I and III
C) II and III
D) II and IV
E) I and II

A) I and IV
B) I and III
C) II and III
D) II and IV
E) I and II
I and III
3
Which statement about bonding in the ammonium ion +NH4 is false?
A) The molecule is tetrahedral.
B) There are four bonding molecular orbitals.
C) There are four antibonding molecular orbitals.
D) All bonding orbitals are occupied.
E) The N hybrid orbitals are made by combining 2 px, 2py , and 2s atomic orbitals.
A) The molecule is tetrahedral.
B) There are four bonding molecular orbitals.
C) There are four antibonding molecular orbitals.
D) All bonding orbitals are occupied.
E) The N hybrid orbitals are made by combining 2 px, 2py , and 2s atomic orbitals.
The N hybrid orbitals are made by combining 2 px, 2py , and 2s atomic orbitals.
4
Which of the following structures is a depiction of structure A?
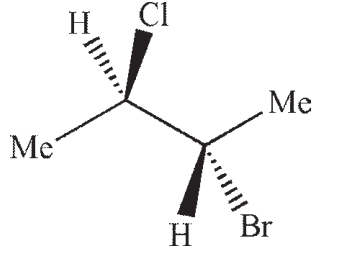
A)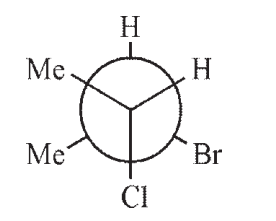
B)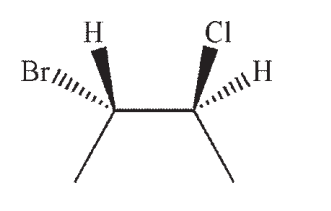
C)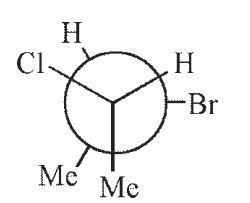
D)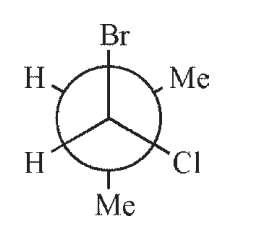
E)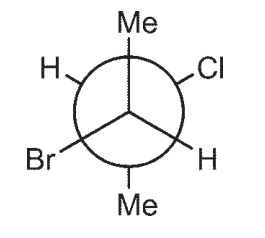

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following compounds is not a constitutional isomer of the others?
A)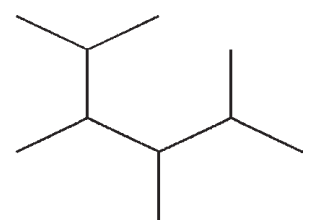
B)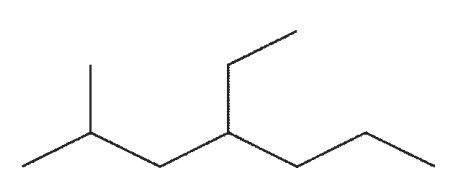
C)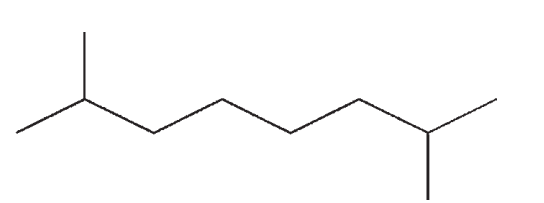
D)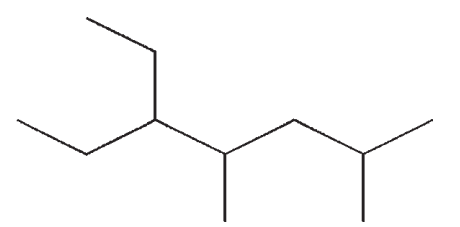
E)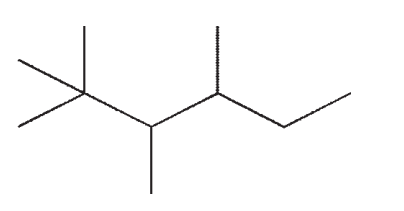
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following statements about methane, CH4 , is false?
A) The carbon-hydrogen bonds in methane are formed by the combination of an sp3 orbital on carbon and a 1s orbital on hydrogen.
B) The CH bonding molecular orbital has cylindrical symmetry.
C) The CH antibonding molecular orbital does not have cylindrical symmetry.
D) The hybrid orbitals on carbon are 25% s character and 75 % p character.
E) All bond angles are 109.5°.
A) The carbon-hydrogen bonds in methane are formed by the combination of an sp3 orbital on carbon and a 1s orbital on hydrogen.
B) The CH bonding molecular orbital has cylindrical symmetry.
C) The CH antibonding molecular orbital does not have cylindrical symmetry.
D) The hybrid orbitals on carbon are 25% s character and 75 % p character.
E) All bond angles are 109.5°.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
7
Which combination of atomic orbitals will produce an sp2 hybrid orbital?
A) 2 px+1 s
B) 2 px+2 s
C) 2 px+2 py+2 pz
D) 2 px+2 pz+2 s
E) 2 px+2 py+2 pz+2 s
A) 2 px+1 s
B) 2 px+2 s
C) 2 px+2 py+2 pz
D) 2 px+2 pz+2 s
E) 2 px+2 py+2 pz+2 s

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following compounds is not a constitutional isomer of the others?
A)
B)
C)
D)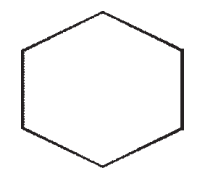
E)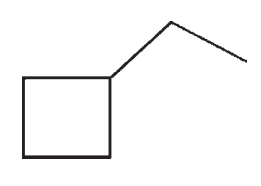
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following choices lists the correct number of each type of carbon atom in the structure shown? 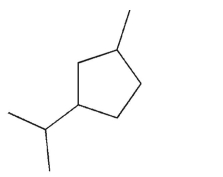
A)3 primary, 3 secondary, 3 tertiary
B)3 primary, 4 secondary, 2 tertiary
C)3 primary, 5 secondary, 1 tertiary
D)5 primary, 1 secondary, 3 tertiary
E)6 primary, 3 secondary, 0 tertiary

A)3 primary, 3 secondary, 3 tertiary
B)3 primary, 4 secondary, 2 tertiary
C)3 primary, 5 secondary, 1 tertiary
D)5 primary, 1 secondary, 3 tertiary
E)6 primary, 3 secondary, 0 tertiary

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following statements about ethane is false?
A) Staggered ethane is destabilized by interactions between filled C-H σ and empty C-H σ* orbitals.
B) Staggered ethane is stabilized by interactions between filled C-H σ and empty C-H σ* orbitals.
C) All staggered conformations are identical in energy, and all eclipsed conformations are identical in energy.
D) The eclipsed conformation of ethane is an energy maximum between staggered conformations.
E) The eclipsed conformation is stabilized by interactions between filled C-H σ bonds.
A) Staggered ethane is destabilized by interactions between filled C-H σ and empty C-H σ* orbitals.
B) Staggered ethane is stabilized by interactions between filled C-H σ and empty C-H σ* orbitals.
C) All staggered conformations are identical in energy, and all eclipsed conformations are identical in energy.
D) The eclipsed conformation of ethane is an energy maximum between staggered conformations.
E) The eclipsed conformation is stabilized by interactions between filled C-H σ bonds.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following structures shows butane in the gauche conformation?
A)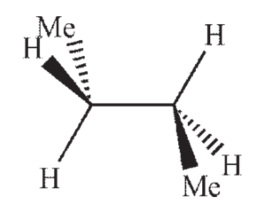
B)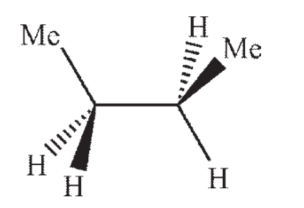
C)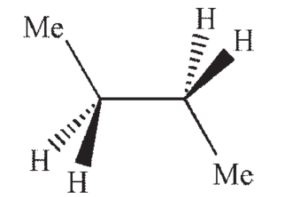
D)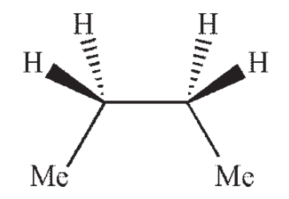
E)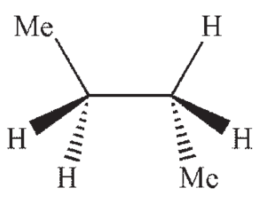
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following structures is not a representation of 2-methylbutane?
A)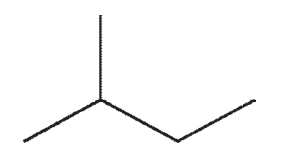
B)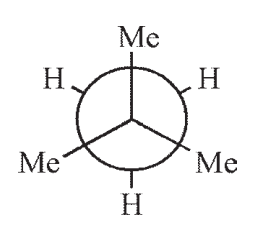
C)
D)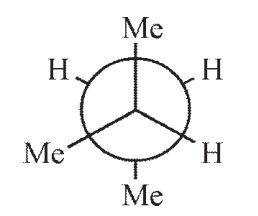
E)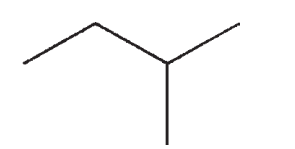
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
13
Which molecule contains a ketone?
A)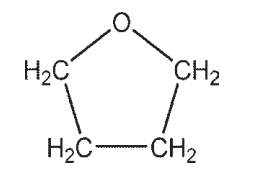
B)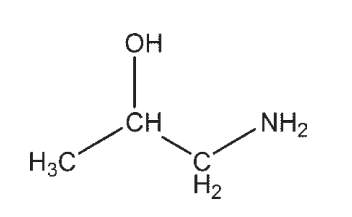
C)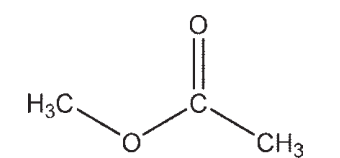
D)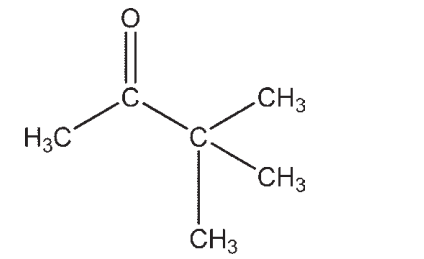
E)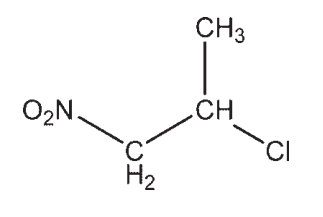
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following Newman projections shows the highest energy conformation of butane?
A)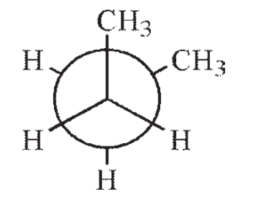
B)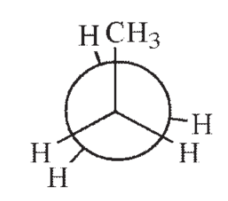
C)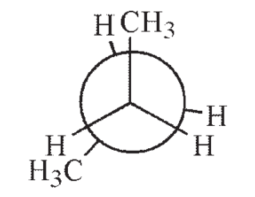
D)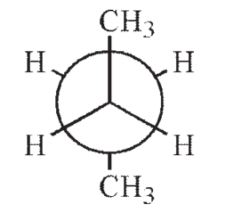
E)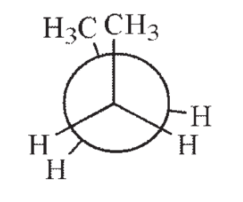
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following molecules contains a quaternary carbon?
A)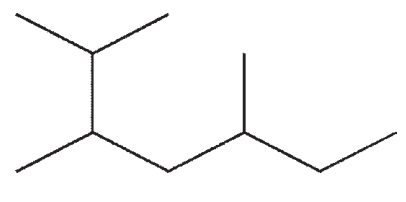
B)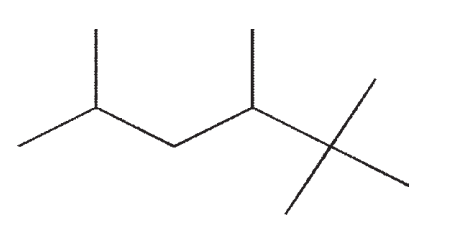
C)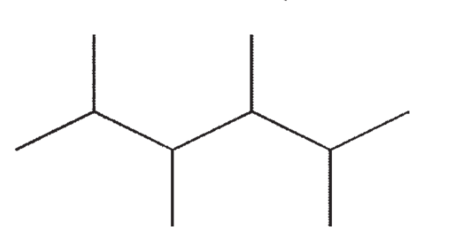
D)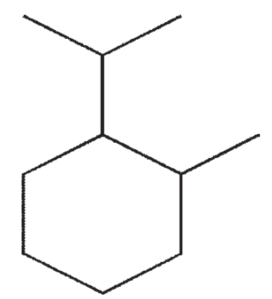
E)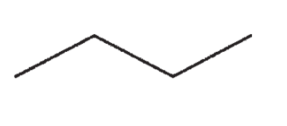
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
16
The quatenary carbon atom in the structure below is atom:
A)I
B)II
C)III
D)IV
E)V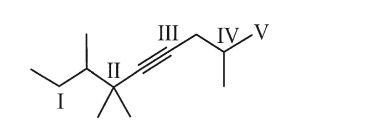
A)I
B)II
C)III
D)IV
E)V


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
17
Which of these structures represent the same compound? 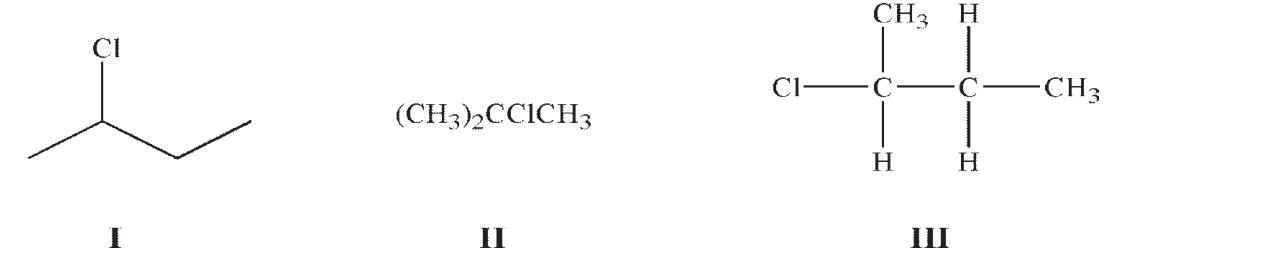
A) I and II
B) I and III
C) II and III
D) I, II, and III
E) They are all different compounds.
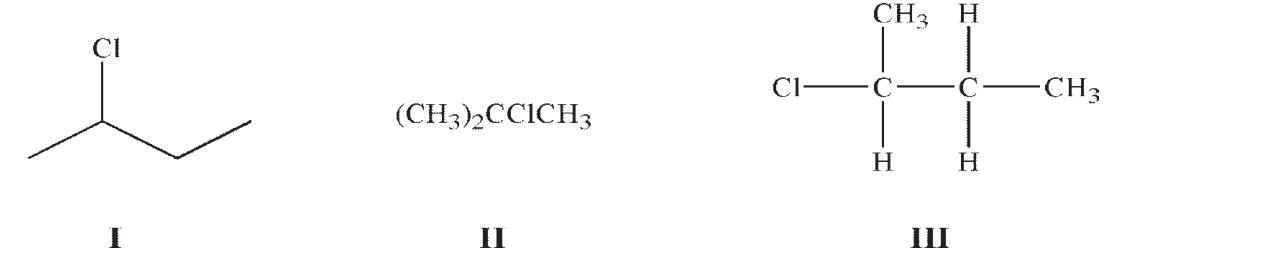
A) I and II
B) I and III
C) II and III
D) I, II, and III
E) They are all different compounds.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
18
Dibromocarbene is an example of a chemical species called a carbene
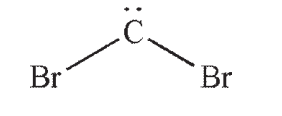
Carbenes exist in one of two forms. In one of these forms, called a singlet, both of the nonbonding electrons on carbon occupy the same orbital. Approximately what type of orbital does the lone pair occupy?
A) sp
B) sp2
C) sp3
D) 2s
E) 2p

Carbenes exist in one of two forms. In one of these forms, called a singlet, both of the nonbonding electrons on carbon occupy the same orbital. Approximately what type of orbital does the lone pair occupy?
A) sp
B) sp2
C) sp3
D) 2s
E) 2p

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following line structures corresponds to the Lewis structure shown here?
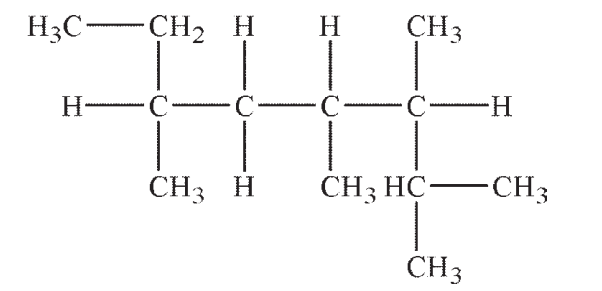
A)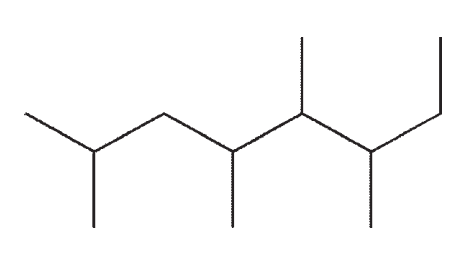
B)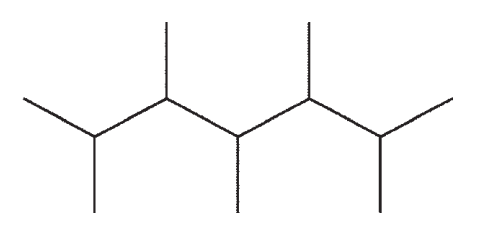
C)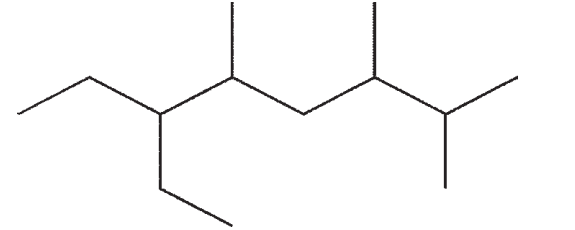
D)
E)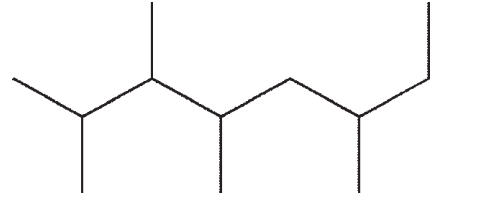

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following Newman projections shows a dihedral angle of 60° between HA and HB?
A)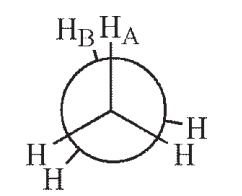
B)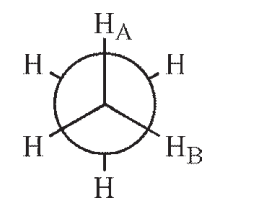
C)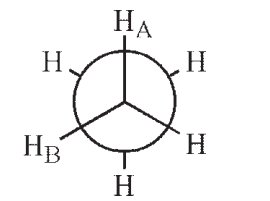
D)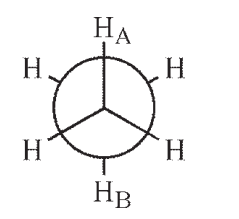
E)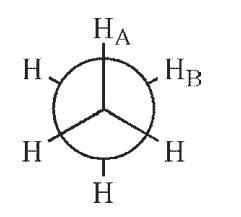
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the Newman projections below depict 3-ethylpentane?
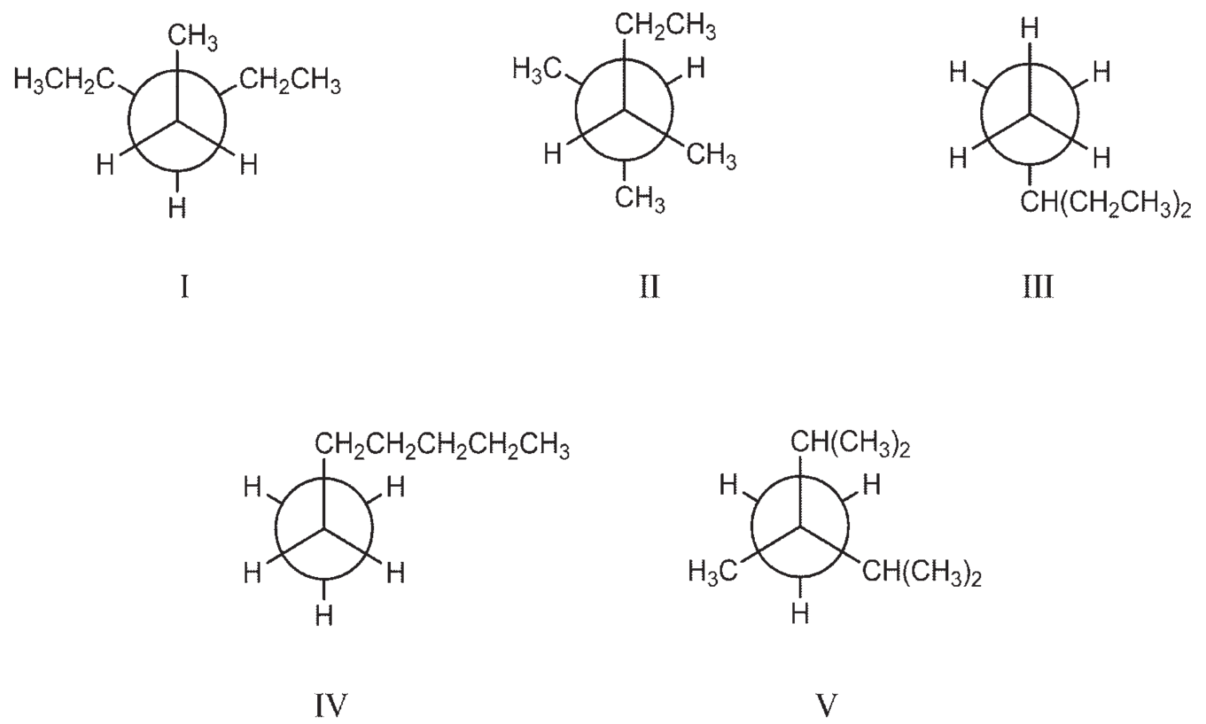
A) I
B) I and II
C) II and III
D) I and III
E) II and IV

A) I
B) I and II
C) II and III
D) I and III
E) II and IV

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following compounds does not have a sp-hybridized carbon atom?
A)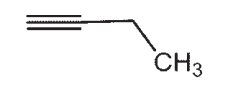
B)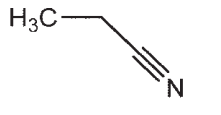
C)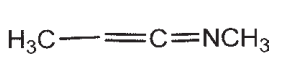
D)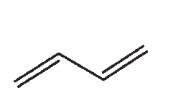
E) CO2
A)

B)

C)
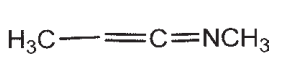
D)

E) CO2

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
23
For the methyl cation +CH3 , indicate which orbitals are involved in the bonding, the hybridization of carbon, the geometry of the cation, and the H-C-H bond angles.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
24
Draw a Newman projection looking down the C2-C3 bond for the lowest energy conformation of 2 -methylbutane.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following structures are saturated hydrocarbons?
A) I and V
B) I, II, and V
C) I, IV, and V
D) III and IV
E) All the structures are saturated hydrocarbons.

A) I and V
B) I, II, and V
C) I, IV, and V
D) III and IV
E) All the structures are saturated hydrocarbons.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
26
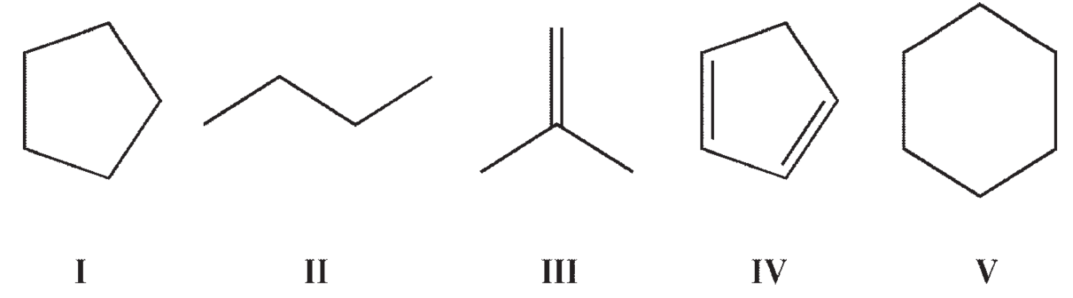
Which of the following structures would show more than two signals on a 1H NMR spectrum?
A)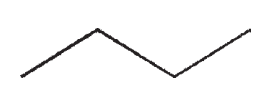
B)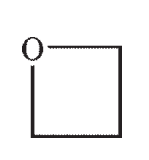
C)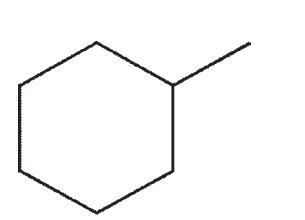
D)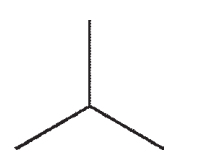
E)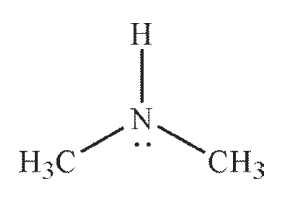
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
27
Which compounds would exhibit three peaks in a 13C NMR spectrum?
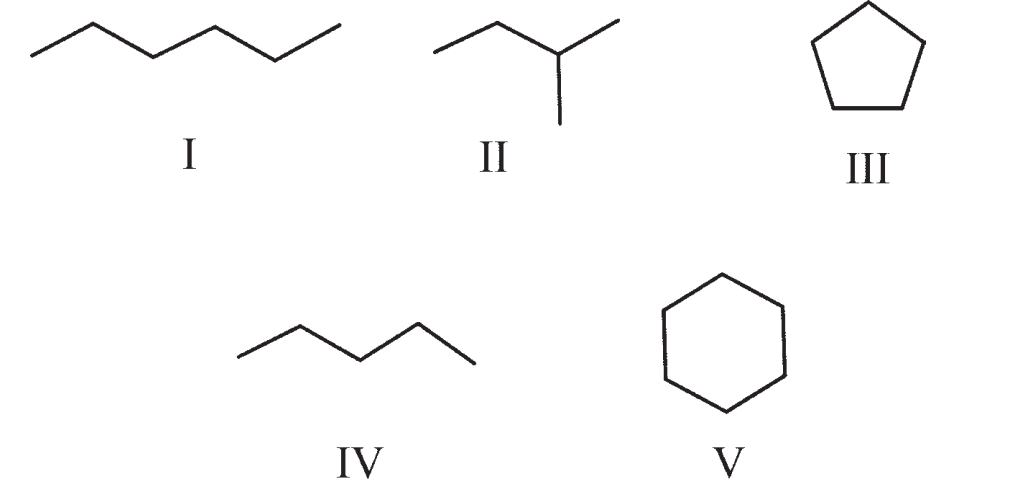
A) III and V
B) II, and IV
C) I and V
D) III and IV
E) I and IV

A) III and V
B) II, and IV
C) I and V
D) III and IV
E) I and IV

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
28
Consider the following set of carbanions: 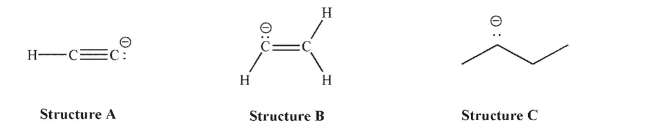 Of the three lone pairs shown, which is most stable (lowest in energy) and why?
Of the three lone pairs shown, which is most stable (lowest in energy) and why?
 Of the three lone pairs shown, which is most stable (lowest in energy) and why?
Of the three lone pairs shown, which is most stable (lowest in energy) and why?
Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following structures is a correct representation of 6-ethyl-5-isopropyl-2,4,7-trimethylnonane?
A)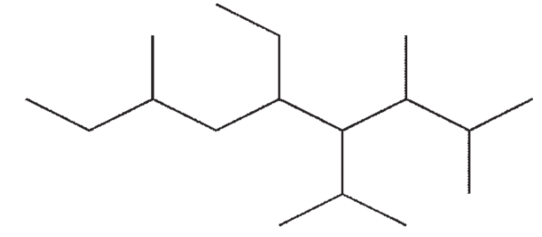
B)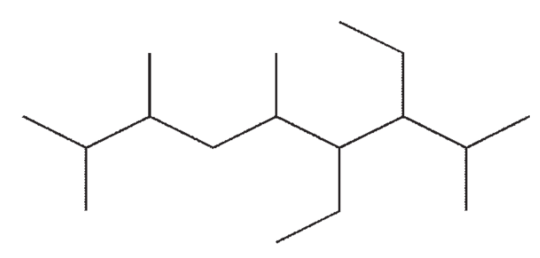
C)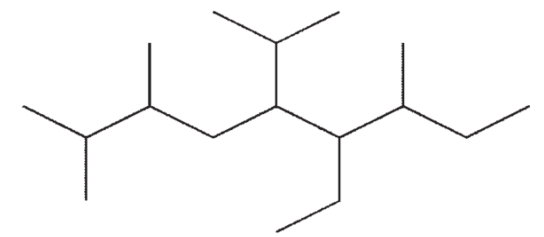
D)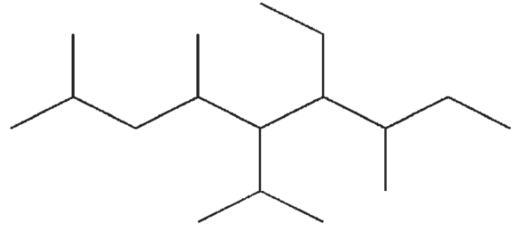
E)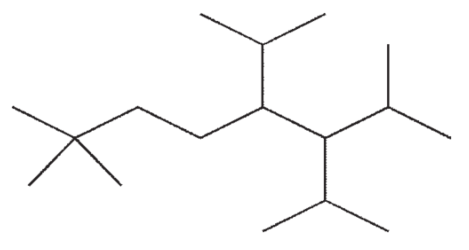
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following statements about chloropropanes is false?
A) There are two signals in the 13C NMR spectrum for 2-chloropropane.
B) There are two signals in the 1H NMR spectrum for 2 -chloropropane.
C) 2-Chloropropane has one constitutional isomer.
D) Looking down the C 1-C 2 bond, all eclipsed conformations of 1-chloropropane are equal in energy.
E) Looking down the C1 - C2 bond, all eclipsed conformations of 2 -chloropropane are equal in energy.
A) There are two signals in the 13C NMR spectrum for 2-chloropropane.
B) There are two signals in the 1H NMR spectrum for 2 -chloropropane.
C) 2-Chloropropane has one constitutional isomer.
D) Looking down the C 1-C 2 bond, all eclipsed conformations of 1-chloropropane are equal in energy.
E) Looking down the C1 - C2 bond, all eclipsed conformations of 2 -chloropropane are equal in energy.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
31
Identify four functional groups in the following molecule. 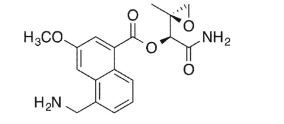


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
32
Consider rotation around the C-1-C-2 single bond of 1-bromopropane. Carefully draw Newman projections of
(A) the two different eclipsed conformations, and
(B) the two different staggered conformations.
(A) the two different eclipsed conformations, and
(B) the two different staggered conformations.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
33
In which of the following molecules can a methyl group be eclipsed either by a chlorine atom or by a bromine atom?
A)1-bromo-3-chloropropane
B)1-bromo-1-chloropropane
C)1-bromo-2-chloropropane
D)2-bromo-1-chloropropane
E)2-bromo-2-chloropropane
A)1-bromo-3-chloropropane
B)1-bromo-1-chloropropane
C)1-bromo-2-chloropropane
D)2-bromo-1-chloropropane
E)2-bromo-2-chloropropane

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following statements is (are) true concerning the three molecules shown?
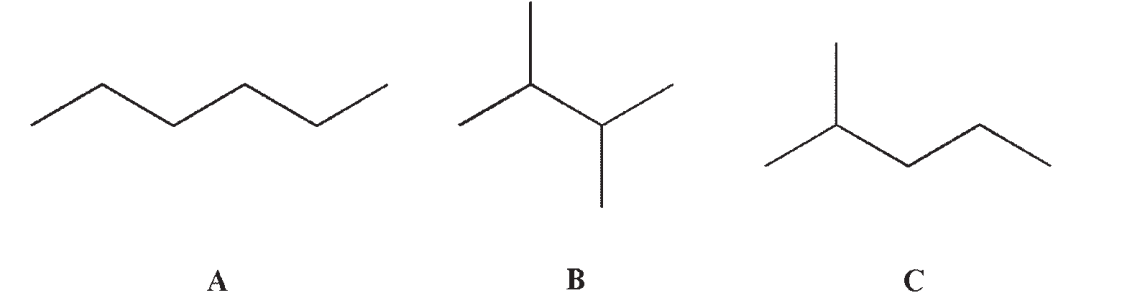
I. The three molecules are constitutional isomers.
II. Structure C is properly named as a hexane.
III. Structure B has the highest boiling point of the three molecules.
IV. Structure A has the highest boiling point of the three molecules.
A) I and II
B) I, II, and III
C) I and III
D) I and IV
E) I only

I. The three molecules are constitutional isomers.
II. Structure C is properly named as a hexane.
III. Structure B has the highest boiling point of the three molecules.
IV. Structure A has the highest boiling point of the three molecules.
A) I and II
B) I, II, and III
C) I and III
D) I and IV
E) I only

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
35
What is the systematic name of this compound?
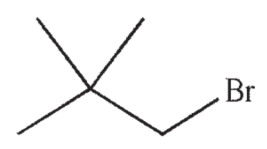
A) 1-bromobutane
B) 1-bromo-2-methylbutane
C) 1-bromo-2-methylpropane
D) 1-bromo-2,2-dimethylpropane
E) 3-bromo-2,2-dimethylpropane

A) 1-bromobutane
B) 1-bromo-2-methylbutane
C) 1-bromo-2-methylpropane
D) 1-bromo-2,2-dimethylpropane
E) 3-bromo-2,2-dimethylpropane

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
36
Provide the hybridization for the indicated atoms in the following compound. 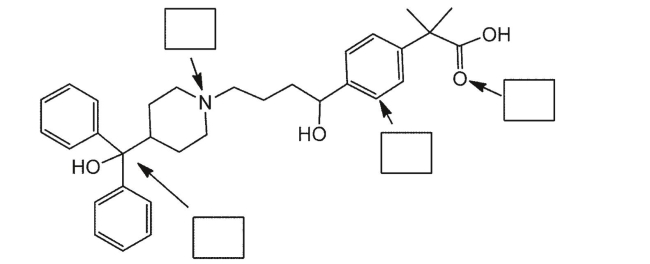


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
37
What orbitals are involved from carbon and hydrogen to form each of the C-H bonds in acetylene?



Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following molecules will have the lowest boiling point?
A)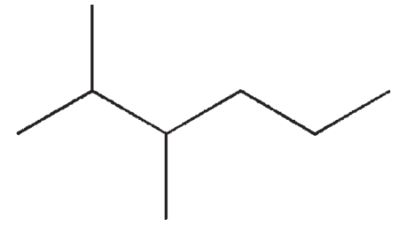
B)
C)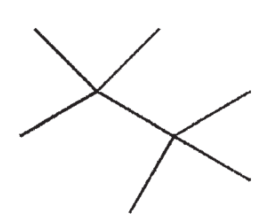
D)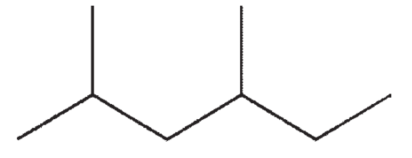
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
39
How many 13C signals will be in an NMR spectrum of the molecule shown?
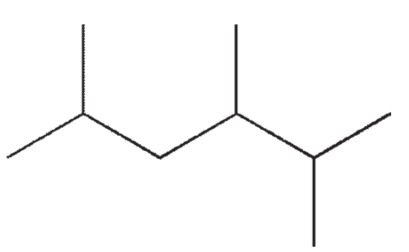
A) 4
B) 5
C) 6
D) 7
E) 9

A) 4
B) 5
C) 6
D) 7
E) 9

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
40
What is the approximate hybridization of each of the indicated carbon atoms in the structures shown here? 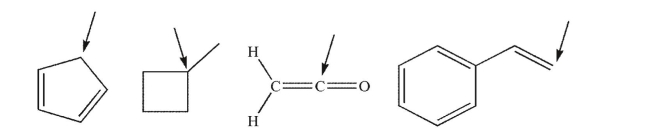


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
41
For compounds like CH3OCH2Cl, it has been determined that the gauche conformation shown here is lower in energy than the anti conformation, a phenomenon called the anomeric effect. One explanation for the anomeric effect is like the one provided for the lower energy of the staggered conformation of ethane relative to eclipsed ethane. This explanation invokes a stabilizing interaction between an empty orbital and a filled orbital in the gauche conformation of the molecule. The Newman projections shown here look down the central C-O bond:
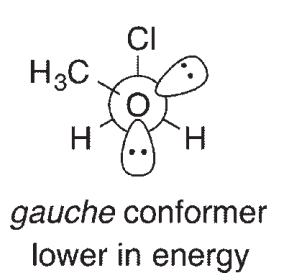
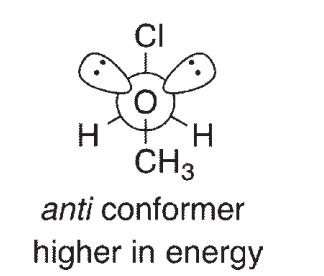
One of the orbitals involved in this theory contains one of the oxygen lone pairs.What is the other
orbital?


One of the orbitals involved in this theory contains one of the oxygen lone pairs.What is the other
orbital?

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
42
The hydrocarbon 2,2,4-trimethylpentane is a popular "antiknock" agent in gasoline.Draw the
Newman projection for the least stable staggered conformer (rotamer) about carbons 3 and 4 of
2,2,4-trimethylpentane.Briefly explain your reasoning.
Newman projection for the least stable staggered conformer (rotamer) about carbons 3 and 4 of
2,2,4-trimethylpentane.Briefly explain your reasoning.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
43
The reaction shown here is one you will encounter later in this course.Identify the Lewis base and the Lewis acid. 


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
44
Draw a structure corresponding to the systematic name 2-Fluoro-3,4,4,6-tetramethylheptane.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
45
Label each atom indicated as primary (1°) , secondary (2°), tertiary (3°), or quaternary (4°).
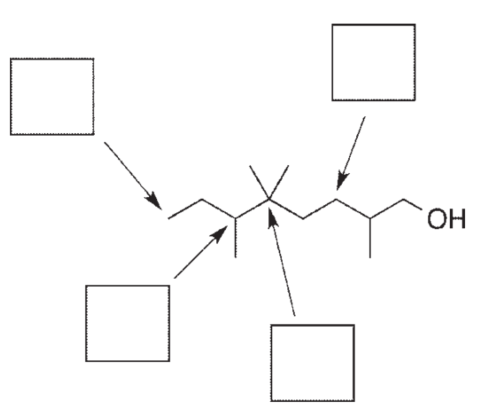


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
46
Write a systematic name for the following compound. 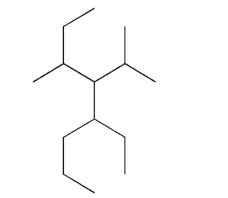


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
47
Draw and provide systematic names for all the constitutional isomers of C4H9Cl where the chlorine atom is attached to a primary carbon.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
48
Draw a line structure and provide the systematic name for sec-butyl chloride.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
49
Draw all constitutional isomers of C7H14 that can be named as cyclopentanes, cyclohexanes, or cycloheptanes. Indicate which of these compounds can exist in cis or trans forms.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
50
Draw Newman projections of all the staggered conformations of butane looking down the C2-C3 bond, and state which of them is the lowest in energy.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
51
Provide a line structure for 2,4,4,5 -tetramethylheptane. In the structure, identify all primary (1°), secondary (2°) , tertiary (3°) , and quaternary (4°) carbons.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
52
Provide the structure for 3-ethyl-4-methyloctane.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
53
Write a systematic name for the following compound. 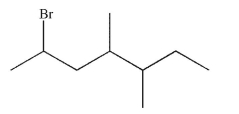


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
54
Draw and provide systematic names for all the constitutional isomers of C7H16 that can be named as pentanes.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
55
How many signals will appear in the 'H NMR spectrum for the molecule shown here? Identify the hydrogen atoms that will generate each signal by labeling them as HA, HB, and so on.
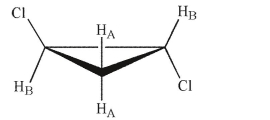


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
56
Draw a molecule with the molecular formula C5H10 that will show four signals in a 13C spectrum.
A)
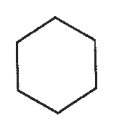
B)
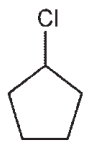
C)
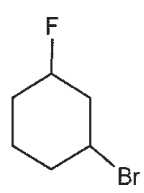
D)
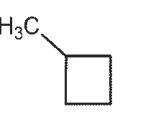
E)
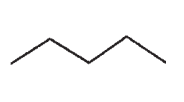
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
57
Arrange the conformations of 1-bromopropane from question 9 (from left to right) in the order most stable to least stable.Using the diagrams of the four conformations, along with brief comment, provide a clear rationale for your ordering of the relative stability of the four conformations.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
58
How many 13C NMR signals would be observed for 3 -ethyl-4-methyloctane?

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
59
Based on the molecular formula C10H18 and using only four-, five-, six-, or seven-membered ring subunits, provide line drawings for
A) two different compounds where two rings do not share any carbons.
B) two different compounds where two rings share one carbon.
C) two different compounds where two rings share two carbons.
A) two different compounds where two rings do not share any carbons.
B) two different compounds where two rings share one carbon.
C) two different compounds where two rings share two carbons.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
60
How many signals will be seen in the 1H NMR spectrum for the following molecule? What will be the relative sizes of these signals? 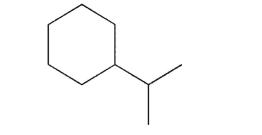


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
61
Using curved arrow formalism, draw a mechanism for the transfer of a proton from acetic acid, CH3COOH, to methylamine, CH3NH2. Draw the products of the reaction and show all lone pairs of electrons and nonzero formal charges.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
62
All Lewis bases may act as Brønsted bases, but not all Lewis acids may act as Brønsted acids.
Explain your answer, providing any examples necessary to support your explanation.
Explain your answer, providing any examples necessary to support your explanation.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
63
Pure water at 25°C undergoes autoionization:

Using curved arrow formalism, draw a mechanism for the transfer of a proton from one water molecule to another to form a hydroxide ion and a hydronium ion. Show all lone pairs of electrons and nonzero formal charges.

Using curved arrow formalism, draw a mechanism for the transfer of a proton from one water molecule to another to form a hydroxide ion and a hydronium ion. Show all lone pairs of electrons and nonzero formal charges.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
64
Tertiary-Butyl cation is an example of a carbocation. 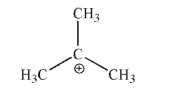 State whether a carbocation is a Lewis acid or a Lewis base, and explain your reasoning.
State whether a carbocation is a Lewis acid or a Lewis base, and explain your reasoning.
 State whether a carbocation is a Lewis acid or a Lewis base, and explain your reasoning.
State whether a carbocation is a Lewis acid or a Lewis base, and explain your reasoning.
Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
65
The reaction shown here is one you will encounter later in this course.Identify the Lewis base and the Lewis acid in the reaction. 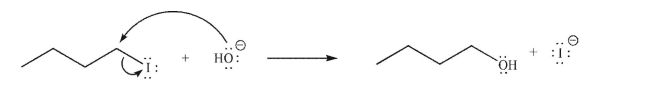


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck



