Deck 17: Carboxylic Acids
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/66
Play
Full screen (f)
Deck 17: Carboxylic Acids
1
Which of the following statements about carboxylic acids is/are true?
A)Carboxylic acids are Brønsted acids.
B)Carboxylic acids are Brønsted bases.
C)Carboxylic acids are Lewis acids.
D)Carboxylic acids are Lewis bases.
E)All these statements are true.
A)Carboxylic acids are Brønsted acids.
B)Carboxylic acids are Brønsted bases.
C)Carboxylic acids are Lewis acids.
D)Carboxylic acids are Lewis bases.
E)All these statements are true.
All these statements are true.
2
Which of these carboxylic acids is most acidic?
A)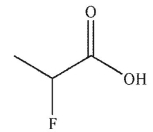
B)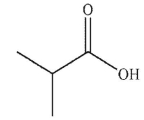
C)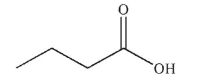
D)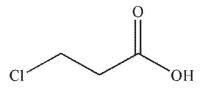
E)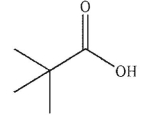
A)

B)

C)

D)

E)


3
Which of these carboxylic acids is in the s-cis conformation?
A)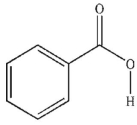
B)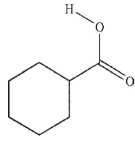
C)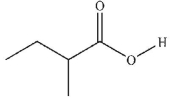
D)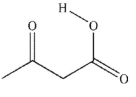
E) both c and d
A)

B)

C)

D)

E) both c and d

4
Which of these structures is 3-pentenoic acid?
A)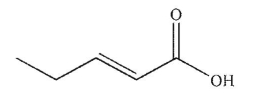
B)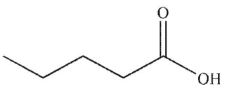
C)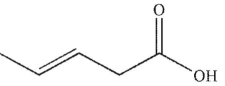
D)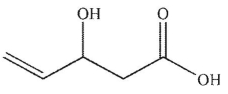
E) None of these structures is 3-pentenoic acid
A)

B)

C)

D)

E) None of these structures is 3-pentenoic acid

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
5
Predict the major organic product of the following reaction.
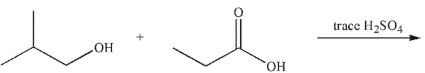
A)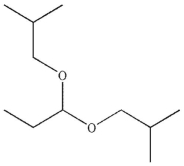
B)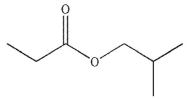
C)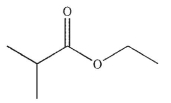
D)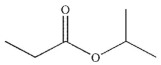
E)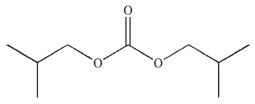

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following compounds corresponds to the spectrum shown here?
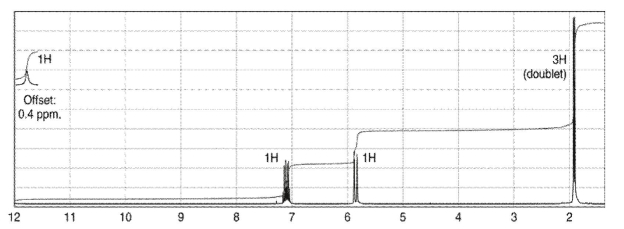
A)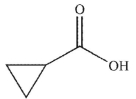
B)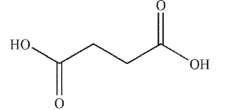
C)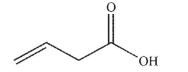
D)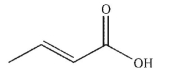
E)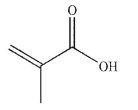

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
7
What is the product of the reaction shown here?
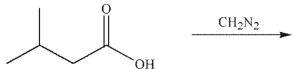
A)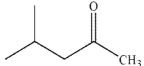
B)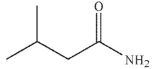
C)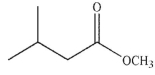
D)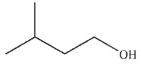
E)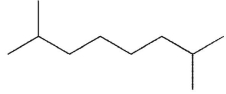

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
8
Which reagent would you use to accomplish the following transformation? 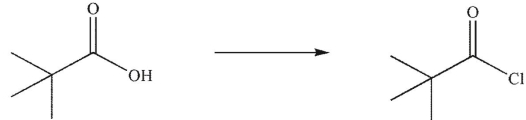
A) HCl
B) Cl2/FeCl3
C) SOCl2
D) Cl2 and heat
E) either b or c

A) HCl
B) Cl2/FeCl3
C) SOCl2
D) Cl2 and heat
E) either b or c

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
9
What reagent would you use to accomplish the following transformation?

A) O3 , then (CH3)2S
B) CH3MgBr, then H3O+
C)HIO4
D) H3O+
E) KMnO4

A) O3 , then (CH3)2S
B) CH3MgBr, then H3O+
C)HIO4
D) H3O+
E) KMnO4

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following statements is true?
A) Carboxylate anions are more susceptible to nucleophilic addition than carboxylic acids.
B) Polarization in the C=O bond destabilizes a carboxylate anion.
C) The carbonyl carbon atom in a carboxylic acid reacts as a Lewis base.
D) Protonation of a carboxylic acid generally occurs faster at the hydroxyl group than at the carbonyl oxygen.
E) Removal of the hydroxyl proton in a carboxylic acid is often the fastest reaction that carboxylic acids undergo.
A) Carboxylate anions are more susceptible to nucleophilic addition than carboxylic acids.
B) Polarization in the C=O bond destabilizes a carboxylate anion.
C) The carbonyl carbon atom in a carboxylic acid reacts as a Lewis base.
D) Protonation of a carboxylic acid generally occurs faster at the hydroxyl group than at the carbonyl oxygen.
E) Removal of the hydroxyl proton in a carboxylic acid is often the fastest reaction that carboxylic acids undergo.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is the correct name for the compound shown here?

A) 2-methyl butanoic acid
B) 2-ethyl propanoic acid
C) (S)-2 -methyl butanoic acid
D) (R)-2 -methyl butanoic acid
E) (S)-2 -ethyl propanoic acid

A) 2-methyl butanoic acid
B) 2-ethyl propanoic acid
C) (S)-2 -methyl butanoic acid
D) (R)-2 -methyl butanoic acid
E) (S)-2 -ethyl propanoic acid

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following compounds corresponds to the spectrum shown here?
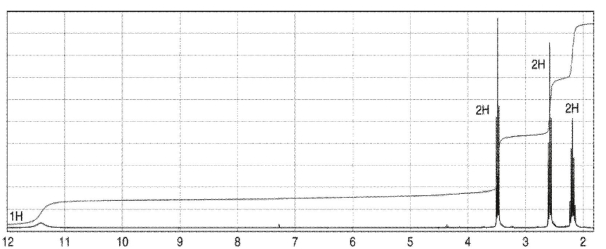
A)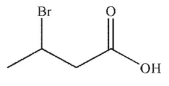
B)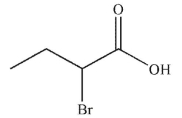
C)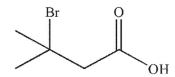
D)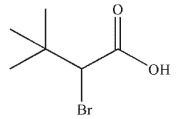
E)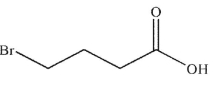

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
13
What is the product of the following reaction? 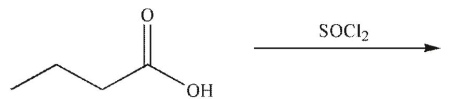
A)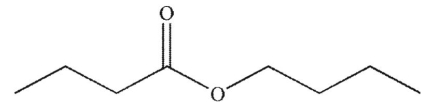
B)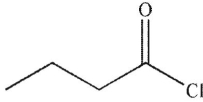
C)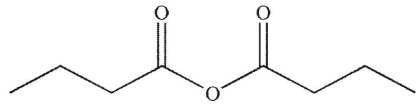
D)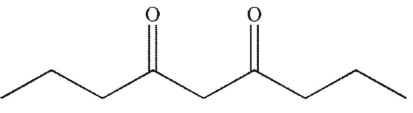
E) No reaction would occur.

A)

B)

C)

D)

E) No reaction would occur.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
14
Which of these structures is not a mechanistic intermediate in acid-catalyzed esterification of a carboxylic acid?
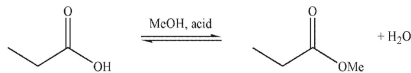
A)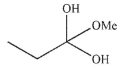
B)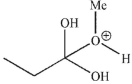
C)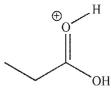
D)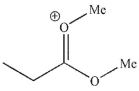
E)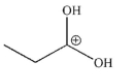
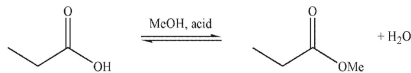
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
15
Which pair of starting materials is needed to make PentexTM polymer, whose structure fragment is shown below?
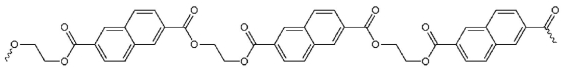
A)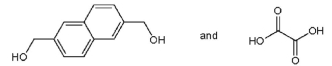
B)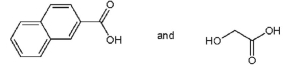
C)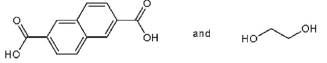
D)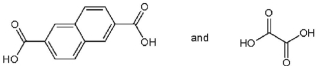
E)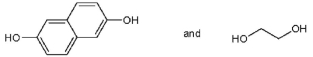

A)

B)
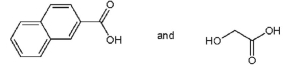
C)

D)

E)
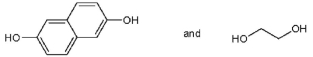

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
16
Place the carboxylic acids shown here in order of increasing acidity.
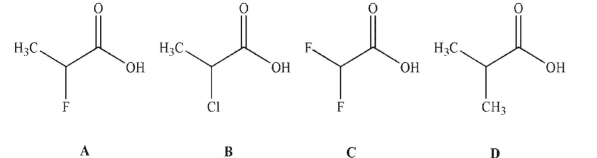
A) C < A < B < D
B) B < D < C < A
C) D < B < C < A
D) D < B < A < C
E) B < D < A < C

A) C < A < B < D
B) B < D < C < A
C) D < B < C < A
D) D < B < A < C
E) B < D < A < C

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following reagents could be used to accomplish the transformation shown here?
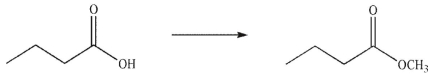
A) CH2N2
B) Methanol and trace H2SO4
C) CH3Li, then H3O+
D) a and b
E) a, b , and c

A) CH2N2
B) Methanol and trace H2SO4
C) CH3Li, then H3O+
D) a and b
E) a, b , and c

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
18
Which starting material(s) is/are needed to make KevlarTM polymer, whose structure fragment is shown below?
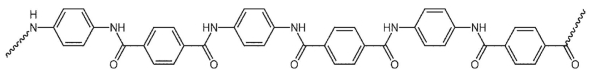
A)
B)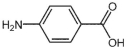
C)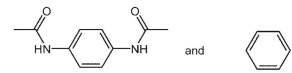
D)
E)

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
19
Predict the product of the following reaction.
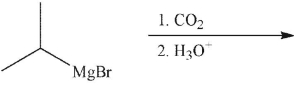
A)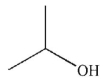
B)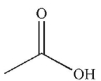
C)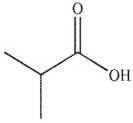
D)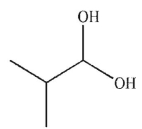
E)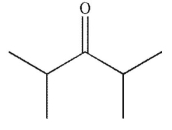
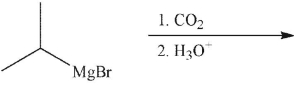
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following reagents could be used to accomplish the following transformation?

A)
B)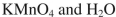
C)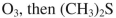
D)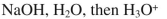
E) either a or b

A)

B)
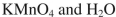
C)
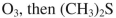
D)
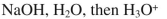
E) either a or b

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
21
What is the product of the following reaction 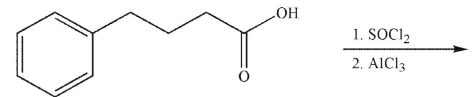
A)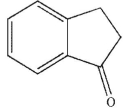
B)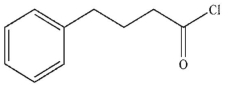
C)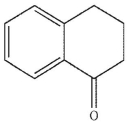
D)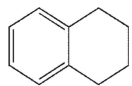
E)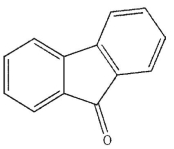

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
22
Explain the difference in acidity between the two acids shown here. 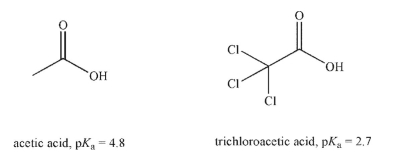


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
23
Starting from benzene, devise two multistep syntheses to make the compound shown here. 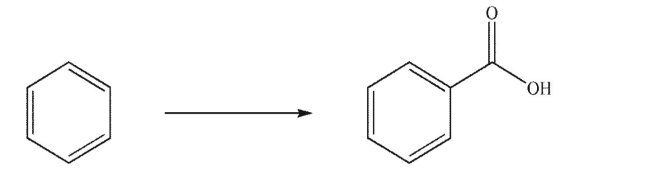


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
24
In the presence of a strong acid, one of the oxygen atoms on the carboxylic acid shown here will become protonated.Which oxygen atom is protonated and why? 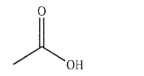


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
25
What is reagent X? 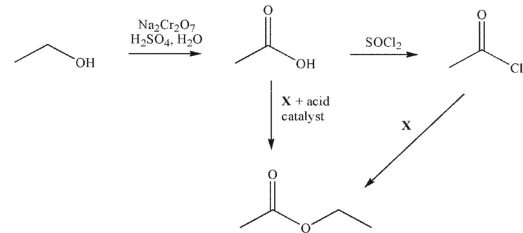
A) CH3CH2Br
B) NaBH4
C) OsO4
D) ethanol
E) H3O+

A) CH3CH2Br
B) NaBH4
C) OsO4
D) ethanol
E) H3O+

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
26
What is the product of the reaction shown here? 
A)
B)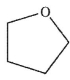
C)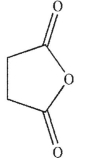
D)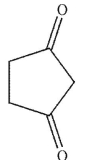
E)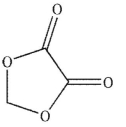

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
27
What is the major organic product of the following reaction? 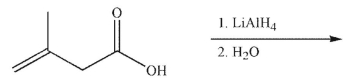
A)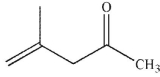
B)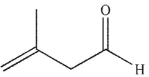
C)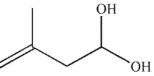
D)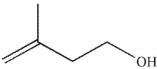
E)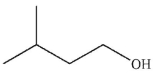

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following structures will not decarboxylate when heated? 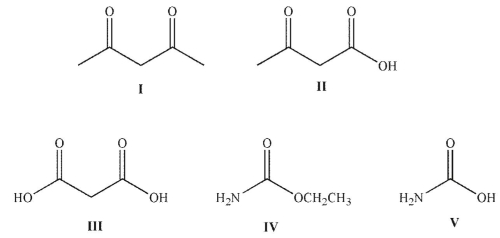
A) I
B) II and III
C) II, III, and IV
D) II, III, and V
E) I and IV

A) I
B) II and III
C) II, III, and IV
D) II, III, and V
E) I and IV

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
29
The reaction shown here is accelerated by using a crown ether called 18-crown-6.Explain why the crown ether has this effect on the reaction. 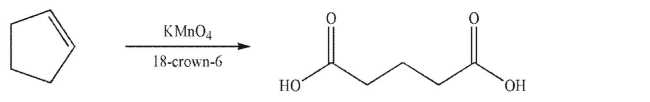


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
30
What is the product of the reaction conditions shown here? 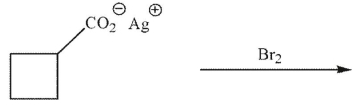
A)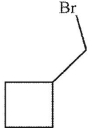
B)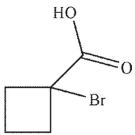
C)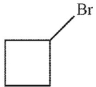
D)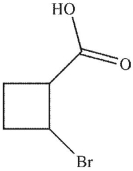
E)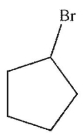
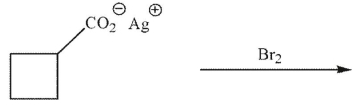
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
31
What are the products of this reaction? 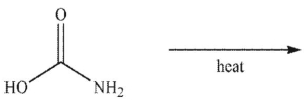
A)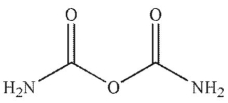
B)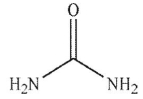
C)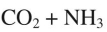
D)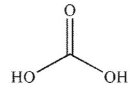
E) The reaction s stable to heating and no reaction will occur.
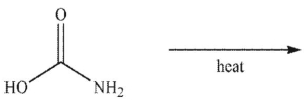
A)

B)

C)
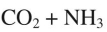
D)

E) The reaction s stable to heating and no reaction will occur.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
32
What is the major organic product of this reaction? 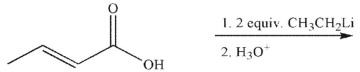
A)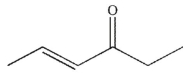
B)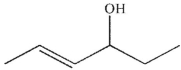
C)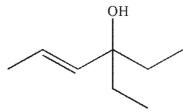
D)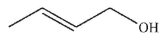
E)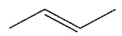
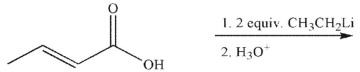
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following compounds do you expect to be unstable?
A)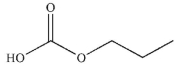
B)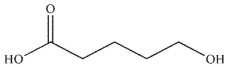
C)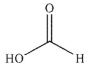
D)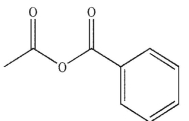
E)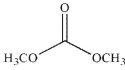
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
34
A compound with a brutto formula C3H4O2 has a sharp IR band at 1690 and a broad IR band at ~3100cm-1 . The 1H NMR spectrum contains three signals: a broad singlet and three multiplets (all doublets of doublets). What is this compound's structure?

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
35
Provide an IUPAC name for the carboxylic acid shown here. 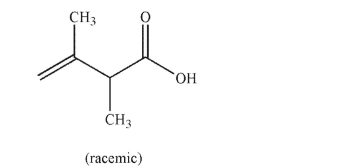


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following compounds could not be the starting material for the acid shown below? 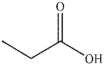
A)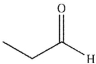
B)
C)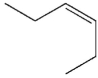
D)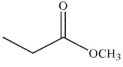
E) The acid could be made from all these compounds.

A)

B)

C)

D)

E) The acid could be made from all these compounds.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
37
What is the major organic product of the following reaction? 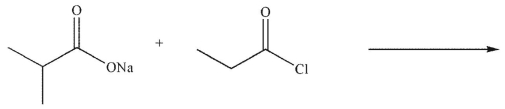
A)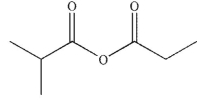
B)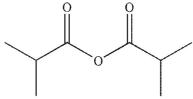
C)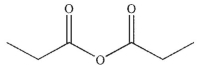
D)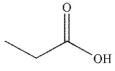
E)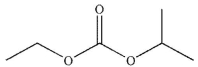

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
38
Which reagent would you use to accomplish the following transformation? 
A) LiAlH4
B) CH3Br, then H3O+
C) 1 equiv. CH3Li, then H3O+
D) 2 equiv. CH3Li, then H3O+
E) CH3OH and trace H2SO4

A) LiAlH4
B) CH3Br, then H3O+
C) 1 equiv. CH3Li, then H3O+
D) 2 equiv. CH3Li, then H3O+
E) CH3OH and trace H2SO4

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
39
What is the major product of the following reaction? 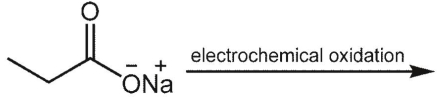
A)CH4
B)C2H6
C)C3H8
D)C4H10
E)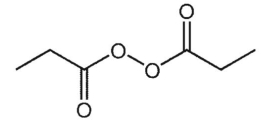

A)CH4
B)C2H6
C)C3H8
D)C4H10
E)


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
40
What is the product of the following sequence of reactions? 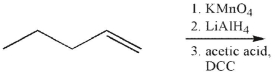
A)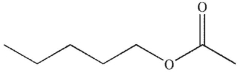
B)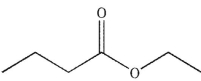
C)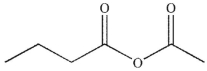
D)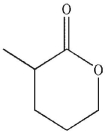
E)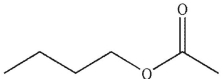

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
41
Draw a mechanism to rationalize the transformation shown here. 


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
42
Draw the structures of the alcohol and carboxylic acid that you would combine to produce the ester shown here. 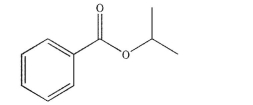


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
43
Isophthaloyl chloride was used as a starting material in a synthesis of the following polymer.What
was the structure of its coupling partner X?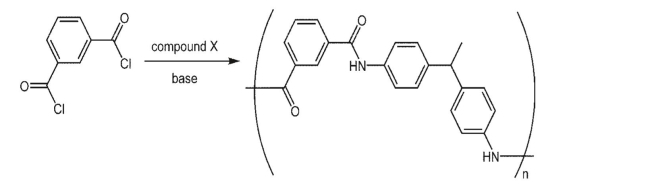
was the structure of its coupling partner X?


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
44
Draw a mechanism for the transformation shown here.Include all necessary lone pairs, curved arrows, and nonzero formal charges. 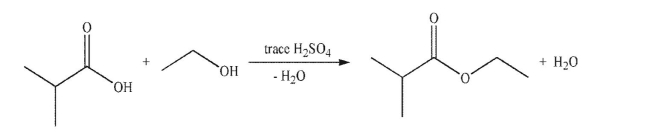


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
45
Predict the major organic product of the following reaction conditions. 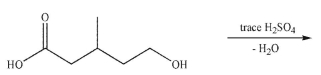


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
46
Draw a mechanism for the following transformation.Include all necessary lone pairs, curved
arrows, and nonzero formal charges.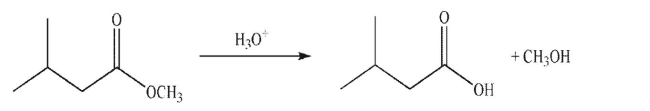
arrows, and nonzero formal charges.


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
47
What is the product of the following reaction? 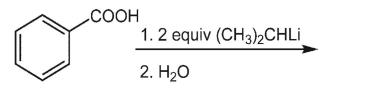


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
48
The compound shown here is the result of a decarboxylation.What is the compound that was
heated to give this product?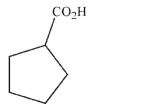
heated to give this product?


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
49
Predict the product of the electrochemical oxidation of the compound shown here. 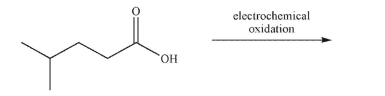


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
50
Heating of the lactam shown below results in a linear polyamide.Draw its structure. 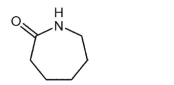


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
51
Draw the structure of polyethylene terephtalate (PET), obtained by reaction below: 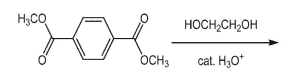


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
52
What is the product of the following reaction? 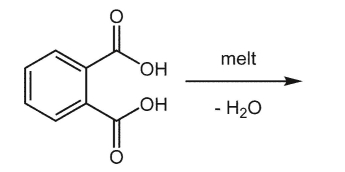


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
53
Design a multistep synthesis of the target molecule from the starting material shown.Show the
reagents needed for each step and the product of each step.You may use any organic or inorganic
reagents, but you may only add a maximum of three carbons to the molecule per step.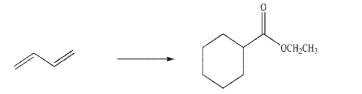
reagents needed for each step and the product of each step.You may use any organic or inorganic
reagents, but you may only add a maximum of three carbons to the molecule per step.


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
54
Indicate how you could form the following ester using a carboxylate ion and an appropriate electrophile. 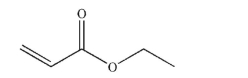


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
55
The reaction shown here is called "iodolactonization." Five-membered rings are usually favored.
Predict the product of this reaction (no need to show stereochemistry).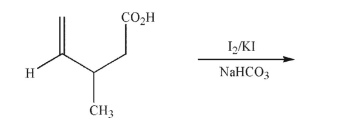
Predict the product of this reaction (no need to show stereochemistry).


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
56
Provide a mechanism for the following transformation.Include all necessary lone pairs, curved arrows, and nonzero formal charges. 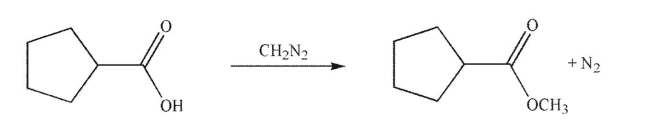


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
57
Predict the product and draw a mechanism for the following transformation. 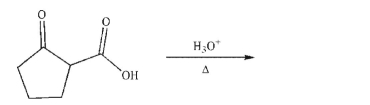


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
58
What reagent can be used to accomplish the following transformation? 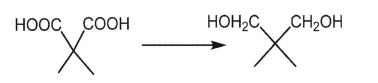


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
59
Draw a mechanism for the following transformation.Include all necessary lone pairs, curved
arrows, and nonzero formal charges.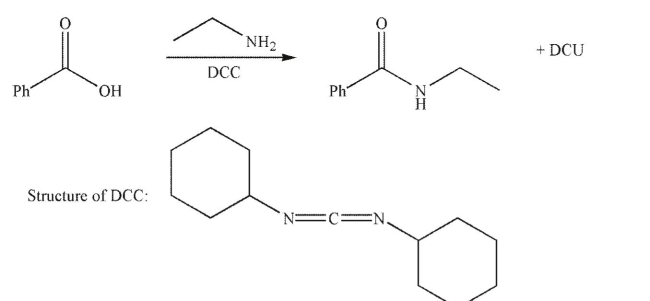
arrows, and nonzero formal charges.


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
60
Draw a mechanism for the following transformation.Include all necessary lone pairs, curved
arrows, and nonzero formal charges.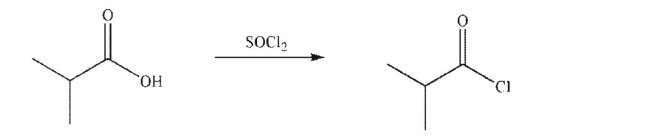
arrows, and nonzero formal charges.


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
61
Design a multistep synthesis of the target molecule from the starting material shown.Show the reagents needed for each step and the product of each step. 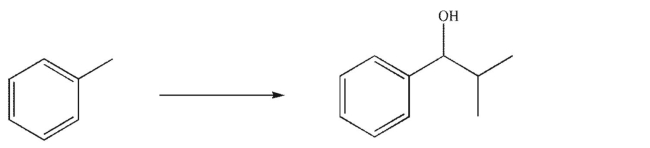


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
62
Explain how soap works.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
63
Predict the major organic product and draw a mechanism for the following transformation.Include
all necessary lone pairs, curved arrows, and nonzero formal charges.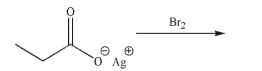
all necessary lone pairs, curved arrows, and nonzero formal charges.


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
64
Suggest a synthesis of 4-pivaloylbenzoic acid from 4-bromotoluene.
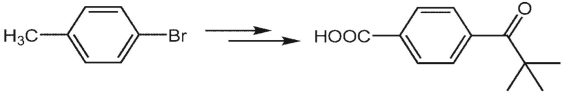
ANS:
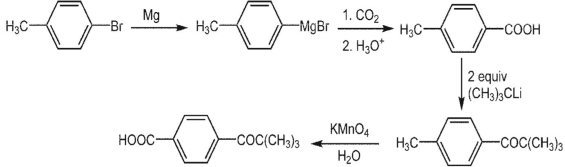

ANS:


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
65
Provide the missing reagents and structures in the following reaction sequence. 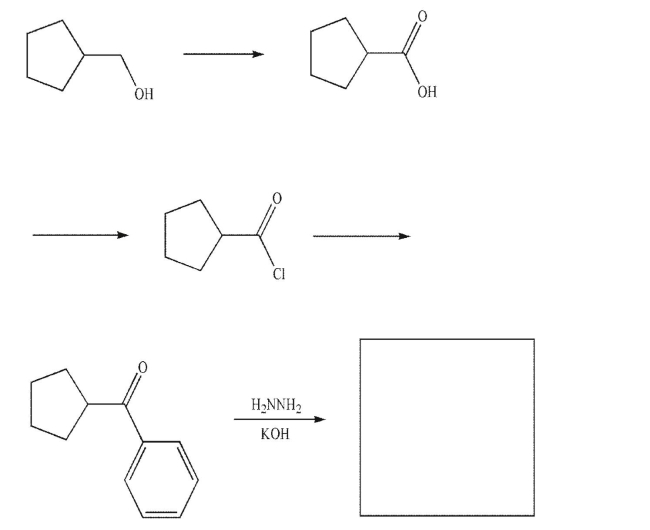


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
66
Design a multistep synthesis of the target molecule from the starting material shown.Show the reagents needed for each step and the product of each step. 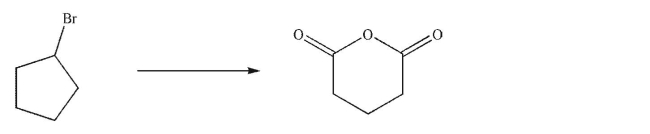


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck



