Deck 14: Aromaticity
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/66
Play
Full screen (f)
Deck 14: Aromaticity
1
Which of these ions is aromatic? Assume all structures are planar.
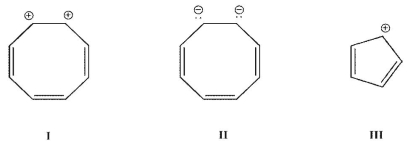
A) I
B) II
C) III
D) I and II
E) I, II, and III

A) I
B) II
C) III
D) I and II
E) I, II, and III
I and II
2
Which of these conditions is not a requirement for aromaticity?
A) planarity
B) (4 n) π
electrons
C) conjugation
D) cyclic structure
E) (4 n+2) π electrons
A) planarity
B) (4 n) π
electrons
C) conjugation
D) cyclic structure
E) (4 n+2) π electrons
(4 n) π
electrons
electrons
3
Which of these ions is aromatic? 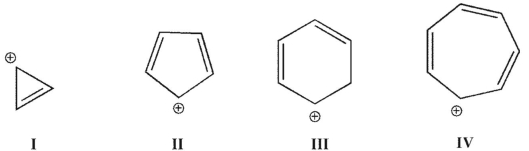
A) I
B) II
C) III
D) II and III
E) I and IV

A) I
B) II
C) III
D) II and III
E) I and IV
I and IV
4
Which of these structures is aromatic? 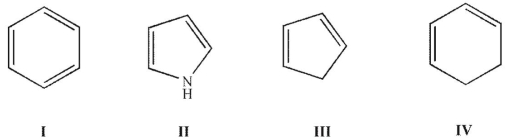
A) I
B) II
C) I and II
D) I, II, and III
E) I, II, and IV

A) I
B) II
C) I and II
D) I, II, and III
E) I, II, and IV

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
5
Which of tiefe following s strucures is cumene?
A)
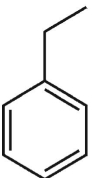
B)
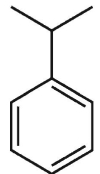
C)
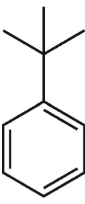
D)
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
6
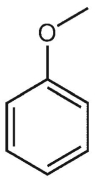
Which of the following structures is anisole?
A)
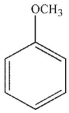
B)
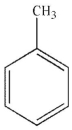
C)

D)
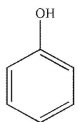
E)
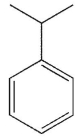
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
7
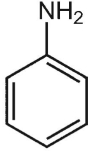
Which of the following structures is aniline?
A)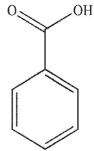
B)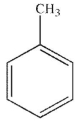
C)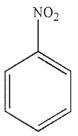
D)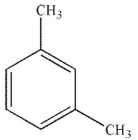
E)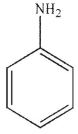
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
8
Which of these ions contains 4n π electrons?
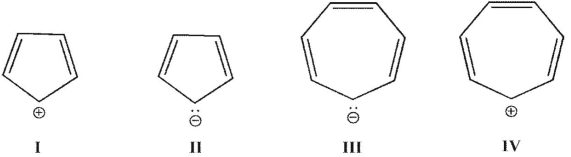
A) I
B) II
C) I and II
D) I and III
E) IV

A) I
B) II
C) I and II
D) I and III
E) IV

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following stucucures is ortho-intoctulene?
A)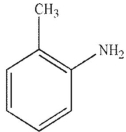
B)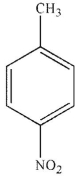
C)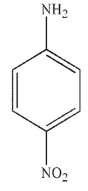
D)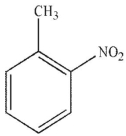
E)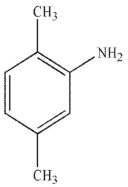
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
10
Which statement about the π molecular orbitals in benzene is true?
A)All antibonding orbitals are occupied.
B)Not all the bonding orbitals are occupied.
C)There are no nonbonding orbitals.
D)All the bonding orbitals have different energies.
E)All the antibonding orbitals have different energies.
A)All antibonding orbitals are occupied.
B)Not all the bonding orbitals are occupied.
C)There are no nonbonding orbitals.
D)All the bonding orbitals have different energies.
E)All the antibonding orbitals have different energies.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following structures is meta-bromoaniline?
A)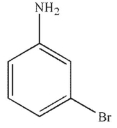
B)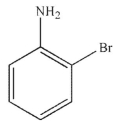
C)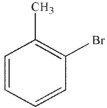
D)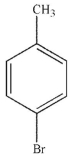
E)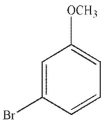
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following structures is aromatic? 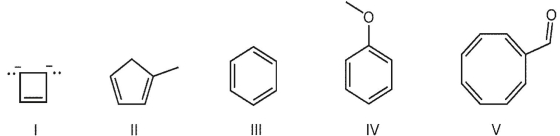
A) I, II, III.
B) I, III, IV.
C) I, II, III, IV.
D) III, IV, V.
E) all of them

A) I, II, III.
B) I, III, IV.
C) I, II, III, IV.
D) III, IV, V.
E) all of them

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following is not aromatic?
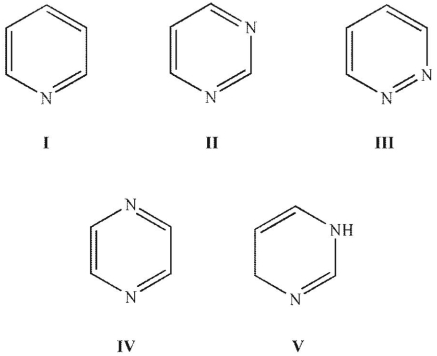
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
14
The heat of hydrogenation of benzene is 
A) equal to 3x the heat of hydrogenation of ethylene.
B) equal to 1.5x the heat of hydrogenation of 1,3 -butadiene.
C) equal to 1.5x the heat of hydrogenation of 1,3 -cyclobutadiene.
D) lower than 3x the heat of hydrogenation of ethylene.
E) higher than 3x the heat of hydrogenation of ethylene.

A) equal to 3x the heat of hydrogenation of ethylene.
B) equal to 1.5x the heat of hydrogenation of 1,3 -butadiene.
C) equal to 1.5x the heat of hydrogenation of 1,3 -cyclobutadiene.
D) lower than 3x the heat of hydrogenation of ethylene.
E) higher than 3x the heat of hydrogenation of ethylene.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
15
With regard to  electrons, which of the following structures is not isoelectronic with the others?
electrons, which of the following structures is not isoelectronic with the others?
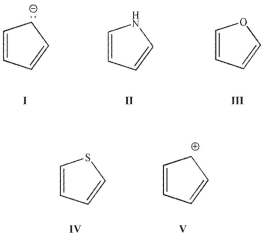
A) I
B) II
C) III
D) IV
E) V
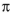 electrons, which of the following structures is not isoelectronic with the others?
electrons, which of the following structures is not isoelectronic with the others?
A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
16
In the bridged structure shown below, what are the most likely 1H NMR chemical shifts (in ppm) for 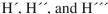 , respectively?
, respectively?
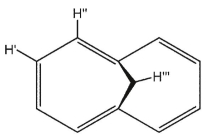
A) 6.95,7.27 , and 6.20
B) 6.95,7.27 , and 4.02
C) 6.95,7.27 , and 0.51
D) 3.02,3.44 , and 0.51
E) 3.02,3.44 , and 6.20
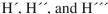 , respectively?
, respectively?
A) 6.95,7.27 , and 6.20
B) 6.95,7.27 , and 4.02
C) 6.95,7.27 , and 0.51
D) 3.02,3.44 , and 0.51
E) 3.02,3.44 , and 6.20

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following statements is false?
A) Benzene undergoes substitution reactions easier than addition reactions.
B) Alternating C-C bonds in benzene have different lengths.
C) Benzene does not undergo Diels-Alder reactions.
D) Hydrogenation of benzene is extremely slow and requires extreme conditions.
E) The double bonds in benzene are conjugated.
A) Benzene undergoes substitution reactions easier than addition reactions.
B) Alternating C-C bonds in benzene have different lengths.
C) Benzene does not undergo Diels-Alder reactions.
D) Hydrogenation of benzene is extremely slow and requires extreme conditions.
E) The double bonds in benzene are conjugated.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
18
Which is a Dewar resonance form of benzene?
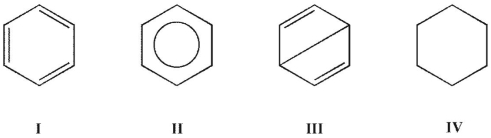
A) I
B) II
C) III
D) IV
E) All these structures may be considered Dewar resonance forms of benzene.

A) I
B) II
C) III
D) IV
E) All these structures may be considered Dewar resonance forms of benzene.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
19
Which of these structures is not aromatic? 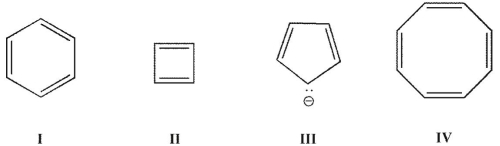
A) I
B) II
C) II and III
D) II, III, and IV
E) II and IV

A) I
B) II
C) II and III
D) II, III, and IV
E) II and IV

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following ions is not aromatic? Assume all structures are planar
A)
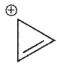
B)
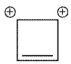
C)
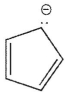
D)
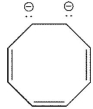
E)
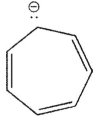
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
21
Is the cis-CH2=CH-CH=CH-CH=CH2 molecule aromatic? Explain why or why not.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
22
Compounds W, X, Y and Z are all alkylbenzenes. W reacts with OsO4 and with NBS. X reacts with NBS , but not OsO4. Y reacts with neither NBS nor OsO4 , and Z reacts with OsO4 but not with NBS . Which of these structures is compound X ?
A)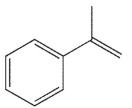
B)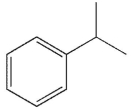
C)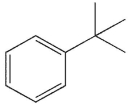
D)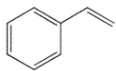
E) None of these
A)

B)

C)

D)

E) None of these

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following compounds has at least one lone pair in an sp2 orbital?
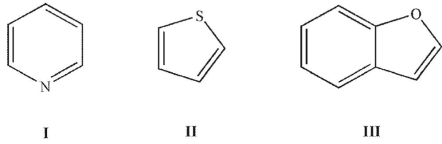
A) I
B) I and II
C) I and III
D) II and III
E) I, II, and III

A) I
B) I and II
C) I and III
D) II and III
E) I, II, and III

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following structures is the product of the reaction shown here? 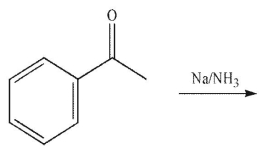
A)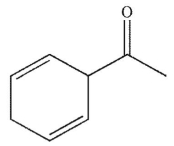
B)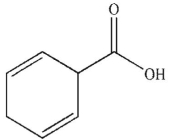
C)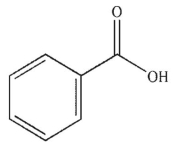
D)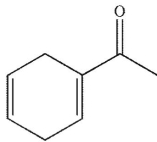
E)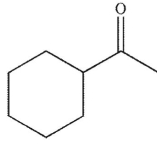

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following will not react with KMnO4?
A)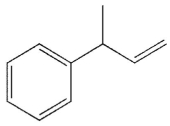
B)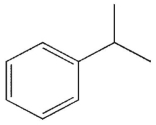
C)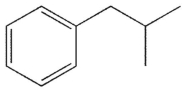
D)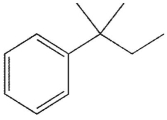
E)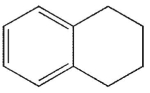
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
26
There are five resonance structures of 1,2-dimethoxycyclopropenyl cation.Draw all of them.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
27
How many isomeric trisubstituted benzenes C3H3XYZ are possible?

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
28
Which of these structures is the major product of the reaction shown?
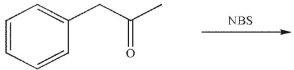
A)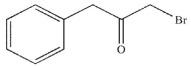
B)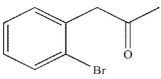
C)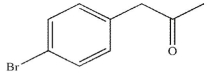
D)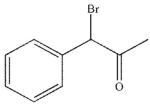
E)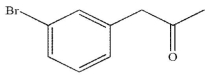

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
29
Which is the correct sequence of reactions required to transform the starting material into the product shown? 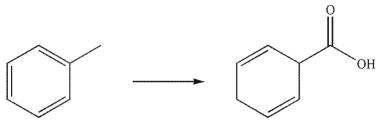
A) Na /NH3, then KMnO4
B) KMnO4,then Na/NH3
C) NBS, then KMnO4
D) KMnO4, NBS, then Na/ NH3
E) Na /NH3, then NBS

A) Na /NH3, then KMnO4
B) KMnO4,then Na/NH3
C) NBS, then KMnO4
D) KMnO4, NBS, then Na/ NH3
E) Na /NH3, then NBS

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following structures is the product of the reaction shown here? 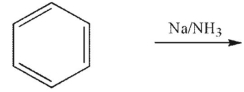
A)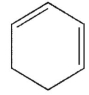
B)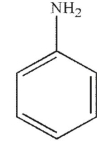
C)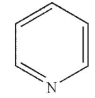
D)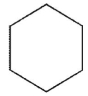
E)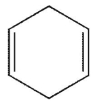

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
31
How many nonbonding π molecular orbitals are there in furan?
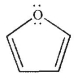
A) 0
B) 1
C) 2
D) 3
E) 5

A) 0
B) 1
C) 2
D) 3
E) 5

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
32
Draw a Frost circle for cyclopentadienyl cation.Include and label all bonding, nonbonding, and antibonding molecular orbitals.Indicate the nonbonding energy level.Put the correct number of electrons in the appropriate orbitals in your drawing.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
33
How many resonances could be observed in the 1H spectrum of Dewar benzene?

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following structures is the product of the reaction shown here? 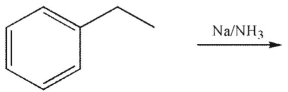
A)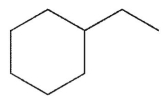
B)
C)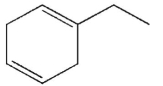
D)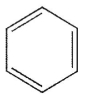
E)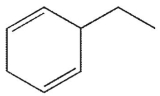

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
35
Why is cyclopentadiene a stronger acid than cycloheptatriene?

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
36
Is the compound shown here aromatic? Explain your answer. 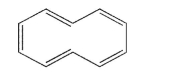


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
37
Place the three molecules shown here in order of increasing acidity.
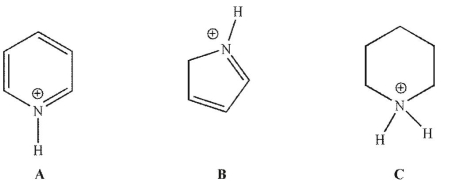
A)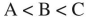
B)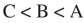
C)
D)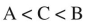
E)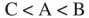

A)
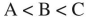
B)
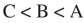
C)
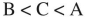
D)
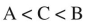
E)
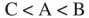

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
38
Fill in the blanks:  species is the least acidic and
species is the least acidic and  species is the most acidic.
species is the most acidic.
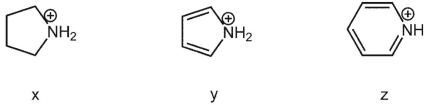
A) x ; z
B) x: y
C) y: z
D) z: y
E) z ; x
 species is the least acidic and
species is the least acidic and  species is the most acidic.
species is the most acidic.
A) x ; z
B) x: y
C) y: z
D) z: y
E) z ; x

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
39
Draw a Frost circle for benzene.Include and label all bonding molecular orbitals and antibonding molecular orbitals.Indicate the nonbonding energy level. Put the correct number of electrons in the appropriate orbitals in your drawing.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
40
Place the three molecules shown here in order of increasing basicity.
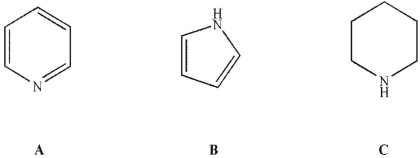
A)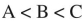
B)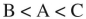
C)
D)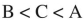
E)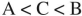

A)
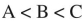
B)
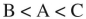
C)
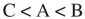
D)
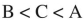
E)
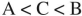

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
41
Devise a multistep synthesis for the following transformation.You may use any organic or inorganic reagents of your choice.Show the reagents necessary for each step and the product of each step. 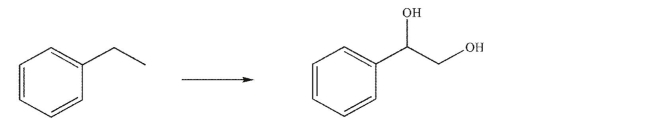


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
42
Is the compound shown here aromatic? Assume planarity. 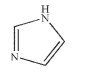


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
43
Draw ortho-methoxytoluene.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
44
Devise a multistep synthesis for the following transformation.You may use any organic or inorganic reagents of your choice.Show the reagents necessary for each step and the product of each step. 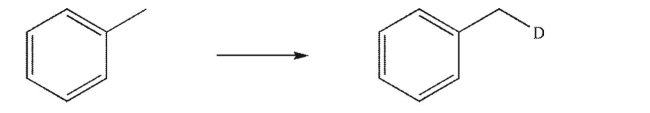


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
45
What is the product of the following reaction? 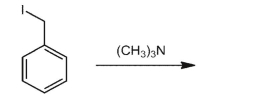


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
46
Draw the product of the following reaction. 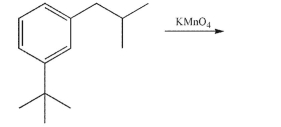


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
47
Draw meta-xylene.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
48
Draw a mechanism for the following transformation.Include all lone pairs of electrons, curved arrows, and formal charges. 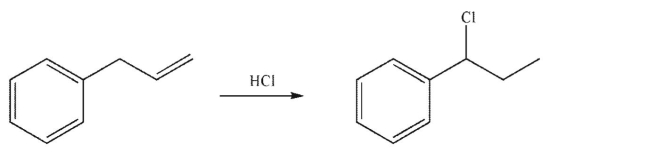


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
49
Which of these two molecules is more reactive in an SN1 reaction, and why?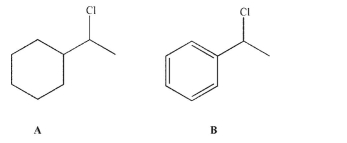


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
50
Draw the product of the following reaction. 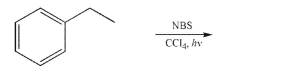


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
51
Draw the structure of styrene.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
52
Devise a multistep synthesis for the following transformation.You may use any organic or inorganic reagents of your choice.Show the reagents necessary for each step and the product of each step. 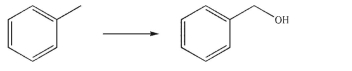


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
53
Three alkylbenzene isomers of C10H14 undergo various reactions. Compound A reacts with KMnO4 to give a para substituted benzoic acid. Compound B does not react with KMnO4. Compound C reacts with KMnO4 to give benzoic acid. Propose structures for compounds A, B , and C .

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
54
Draw a Frost circle for pyrrole.Include and label all bonding molecular orbitals and antibonding molecular orbitals.Indicate the nonbonding energy level.Put the correct number of electrons in
the appropriate orbitals in your drawing.
the appropriate orbitals in your drawing.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
55
Which nitrogen in imidazole is more basic? Explain your answer. 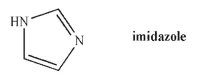
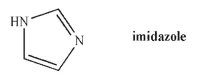

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
56
In furan, what type of orbital do each of the two lone pairs on oxygen occupy?

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
57
Draw the structure of toluene.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
58
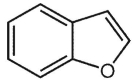
The compound below is called benzofuran. Suggest an isomeric structure containing fused 5 -membered heterocycle. Does it obey the 4n +2 rule?


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
59
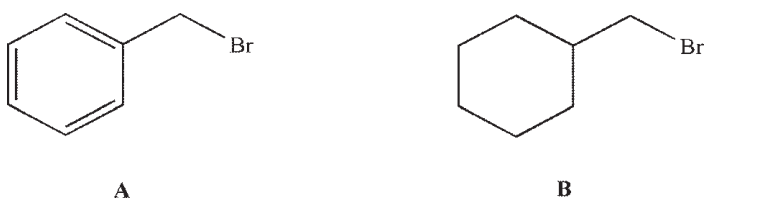
Which of these two molecules is more reactive in an SN2 reaction, and why?


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
60
Draw the structures of the following compounds and place them in the order of increasing boiling points: toluene, p-ethyltoluene, p-ethylbenzoic acid, p-diethylbenzene.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
61
Draw an arrow-pushing mechanism for the following transformation.Include all lone pairs and single electrons, curved arrows, and formal charges.Indicate the relative rate of each step. 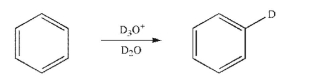


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
62
Draw a complete mechanism for the following transformation.Include all lone pairs and single
electrons, curved arrows, and formal charges.Include all significant resonance structures.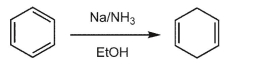
electrons, curved arrows, and formal charges.Include all significant resonance structures.


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
63
Devise a multistep synthesis for the following transformation.You may use any organic or inorganic reagents of your choice.Show the reagents necessary for each step and the product of each step. 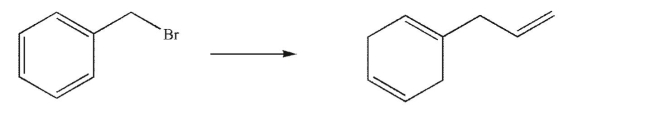


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
64
Draw the product of the following reaction. 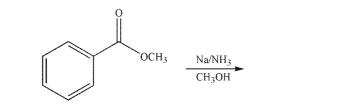


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
65
Devise a multistep synthesis for the following transformation.You may use any organic or inorganic reagents of your choice.Show the reagents necessary for each step and the product of each step. 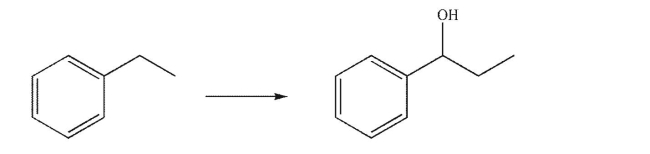


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
66
Draw the product of the following reaction. 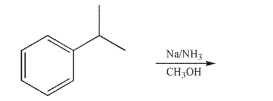


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck



