Deck 19: Carbonyl Chemistry 2: Reactions at the Α Position
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/71
Play
Full screen (f)
Deck 19: Carbonyl Chemistry 2: Reactions at the Α Position
1
Which of the following could not serve as a starting material in the synthesis of the following material?
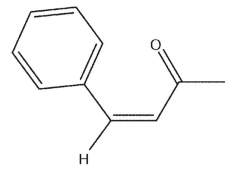
A)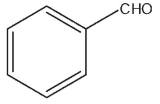
B)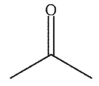
C)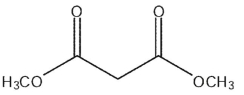
D)
E)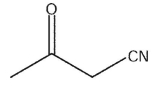

A)

B)

C)

D)

E)


2
Which of the following compounds is the weakest acid?
A)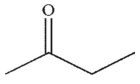
B)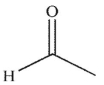
C)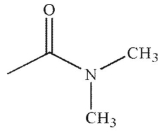
D)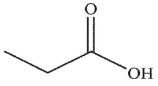
E)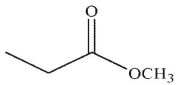
A)

B)

C)

D)

E)


3
Which of the following is the correct product of the reaction sequence shown? 
A)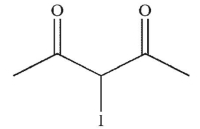
B)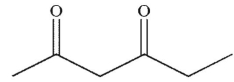
C)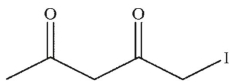
D)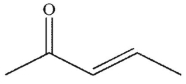
E)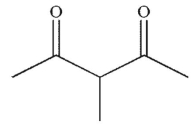

A)

B)

C)

D)

E)


4
Which of the following compounds could not be used as a substrate for an alkylation reaction with a ketone enolate?
A)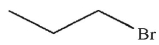
B)
C)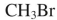
D)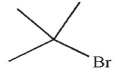
E)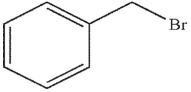
A)

B)

C)
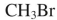
D)

E)


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
5
Rank the circled protons in the following compounds from least acidic to most acidic: 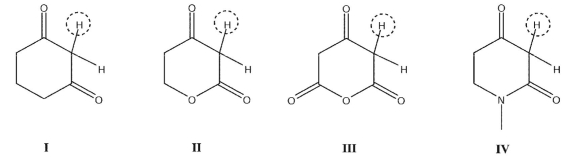
A)
B)
C)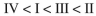
D)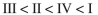
E)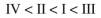

A)

B)

C)
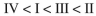
D)
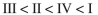
E)
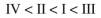

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
6
Why is the carbon atom typically the nucleophilic site of an enolate anion? 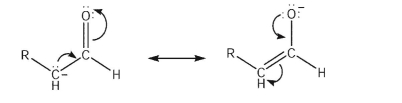
A)Most of the negative charge resides on the carbon atom.
B)The resonance form with the negative charge on carbon is the major contributor.
C)The alkoxy anion will not act as a Lewis base, due to oxygen electronegativity.
D)The HOMO of the enolate has its largest lobe at the beta-carbon.
E)The bonding MO of the enolate has its highest electron density around the oxygen.

A)Most of the negative charge resides on the carbon atom.
B)The resonance form with the negative charge on carbon is the major contributor.
C)The alkoxy anion will not act as a Lewis base, due to oxygen electronegativity.
D)The HOMO of the enolate has its largest lobe at the beta-carbon.
E)The bonding MO of the enolate has its highest electron density around the oxygen.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is the correct product of the reaction conditions shown? 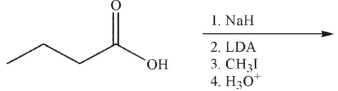
A)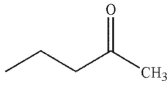
B)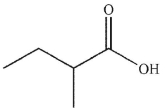
C)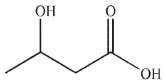
D)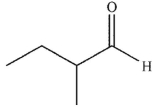
E)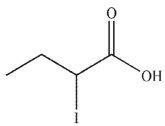

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
8
Why should lithium diisopropylamide (LDA) be used in enolate alkylation reactions?
A)LDA completely converts one equivalent of carbonyl to enolate.
B)LDA is too bulky to act as a nucleophile.
C)Lithium complexation with the enolate oxygen can reduce O-alkylation.
D)LDA will selectively remove the least hindered protons from the carbonyl.
E)All these reasons are valid.
A)LDA completely converts one equivalent of carbonyl to enolate.
B)LDA is too bulky to act as a nucleophile.
C)Lithium complexation with the enolate oxygen can reduce O-alkylation.
D)LDA will selectively remove the least hindered protons from the carbonyl.
E)All these reasons are valid.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
9
What is the final product of the following reaction sequence? 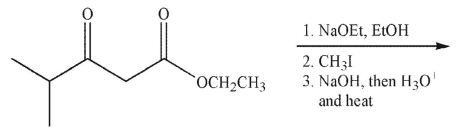
A)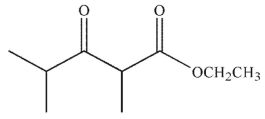
B)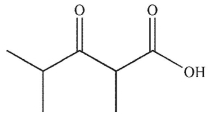
C)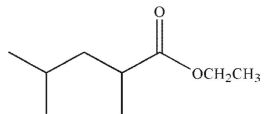
D)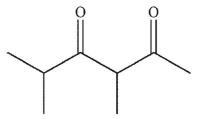
E)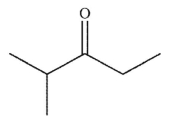

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following structures will have the greatest amount of enol content at equilibrium?
A)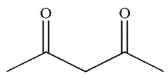
B)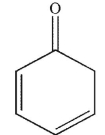
C)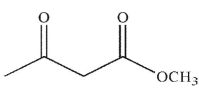
D)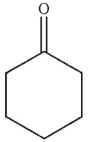
E)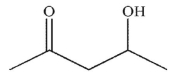
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is the correct product of the reaction conditions shown? 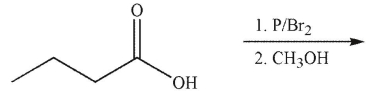
A)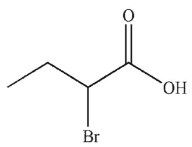
B)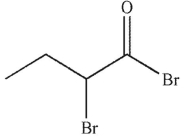
C)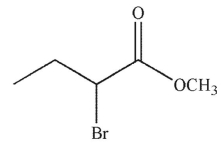
D)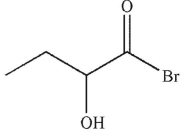
E)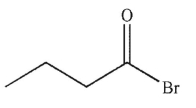

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
12
What is the final product of the following reaction sequence? 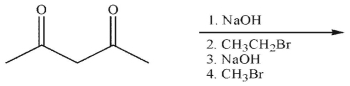
A)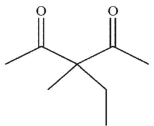
B)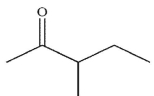
C)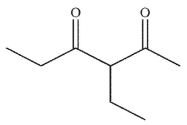
D)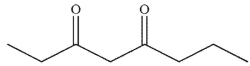
E)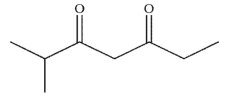
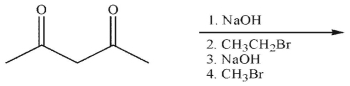
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
13
What is the product of the following transformation? 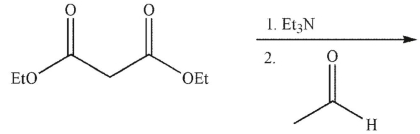
A)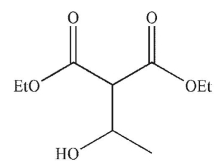
B)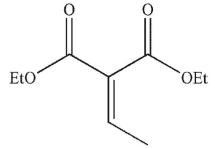
C)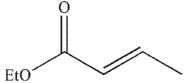
D)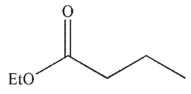
E) Either a or b is possible.

A)

B)

C)

D)

E) Either a or b is possible.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
14
What are the starting materials needed to make the molecule shown using an aldol condensation? 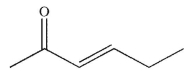
A)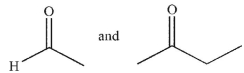
B)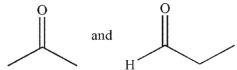
C)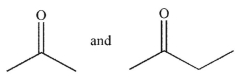
D)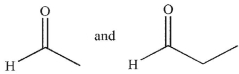
E)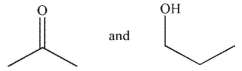

A)

B)

C)

D)

E)
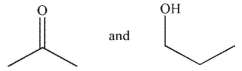

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
15
What would be the expected product of the following synthetic sequence? 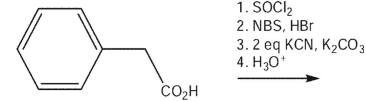
A)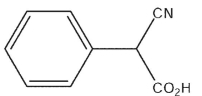
B)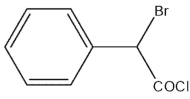
C)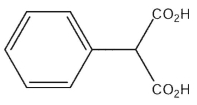
D)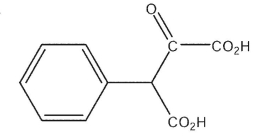
E)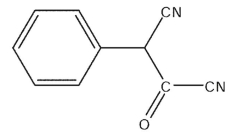

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
16
What is the product of the reaction conditions shown?
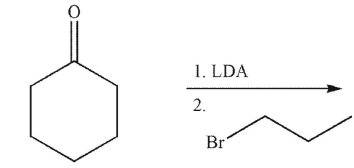
A)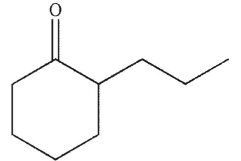
B)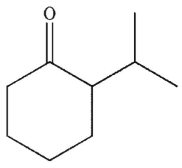
C)
D)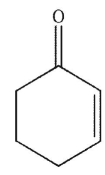
E)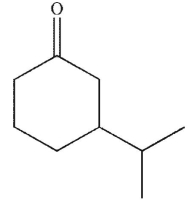

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
17
What is the final product of this reaction sequence? 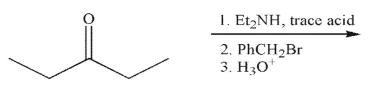
A)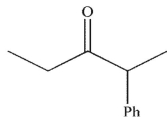
B)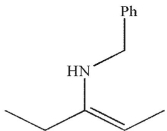
C)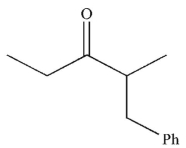
D)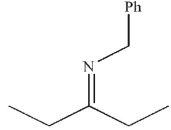
E)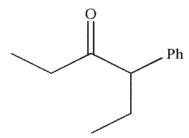
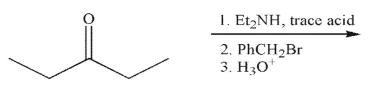
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
18
Predict the major organic product of the following reaction. 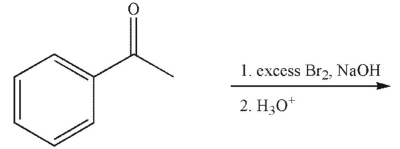
A)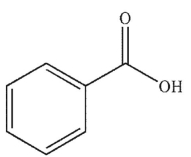
B)
C)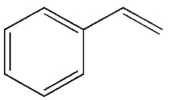
D)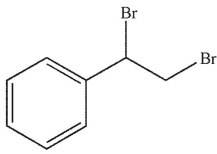
E)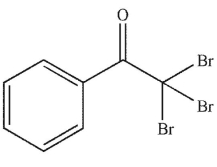
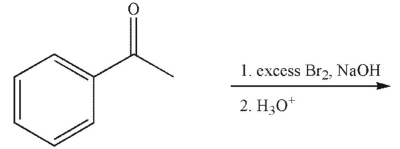
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is the correct product of the conditions shown?
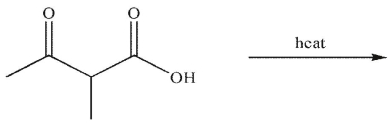
A)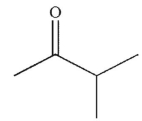
B)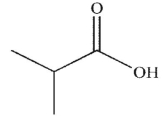
C)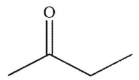
D)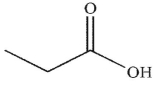
E)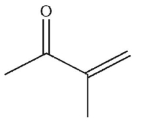

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
20
What is the product of this reaction? 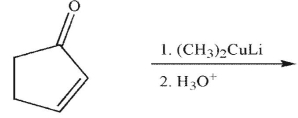
A)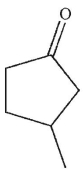
B)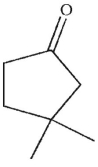
C)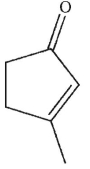
D)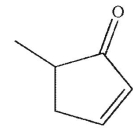
E)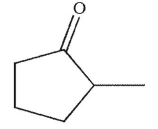
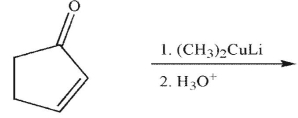
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following is a thermodynamic enolate?
A)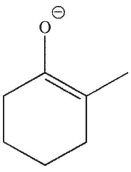
B)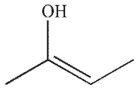
C)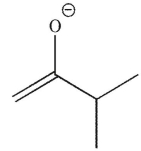
D)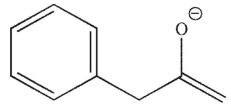
E)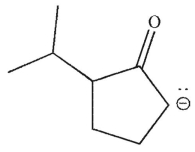
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
22
In aqueous base, the chiral ketone shown here racemizes.Provide an explanation. 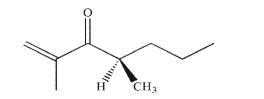


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
23
What is the product of this reaction?
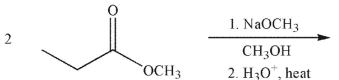
A)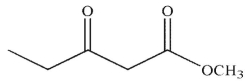
B)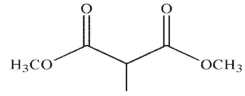
C)
D)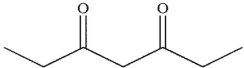
E)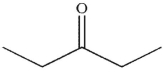

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
24
Which of these structures is the product of the reaction? 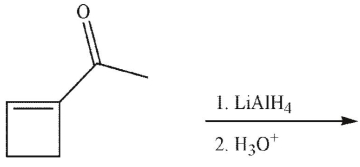
A)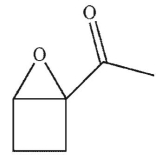
B)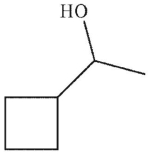
C)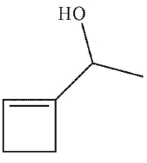
D)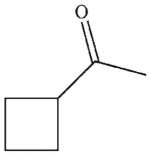
E) None of these structures is the product of the reaction.
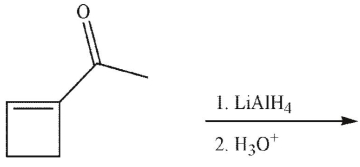
A)

B)

C)

D)

E) None of these structures is the product of the reaction.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
25
Provide a mechanism for the following transformation. 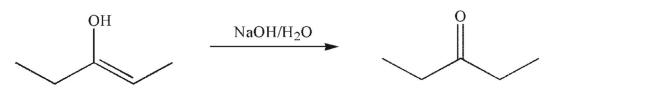


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
26
Which of these compounds is the stronger acid? Explain your reasoning. 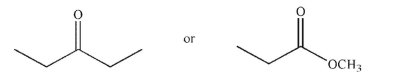


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following would be products of the following crossed aldol condensation reaction? 
A)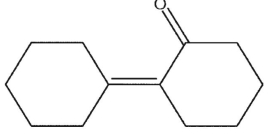
B)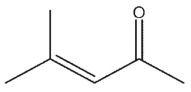
C)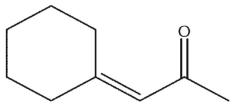
D)
E) All would be products

A)

B)

C)

D)

E) All would be products

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
28
What would be the product of the following reaction sequence?
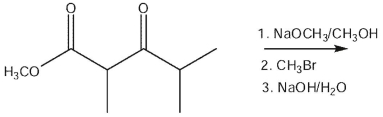
A)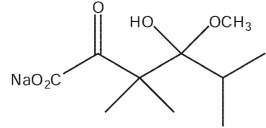
B)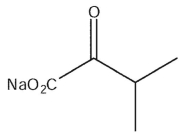
C)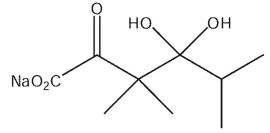
D)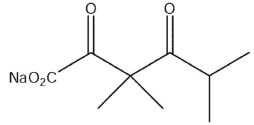
E)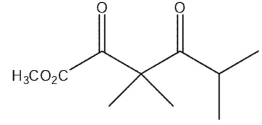

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
29
What is the product of the following reaction sequence? 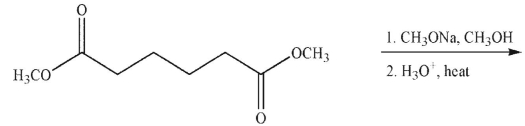
A)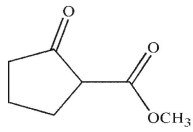
B)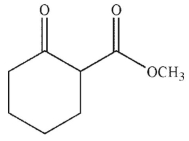
C)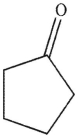
D)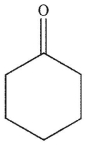
E)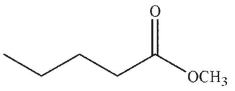

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
30
What is the product of the following reaction sequence? 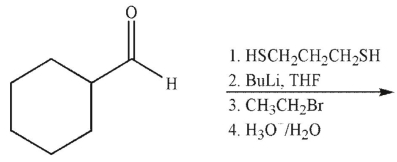
A)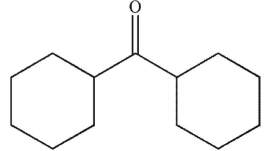
B)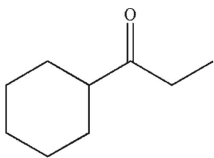
C)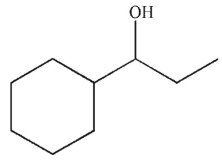
D)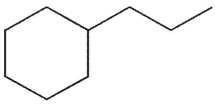
E)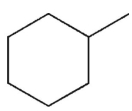

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following compounds cannot undergo a Dieckmann condensation?
A)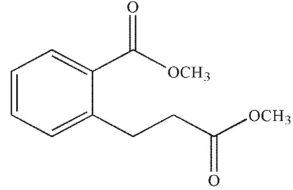
B)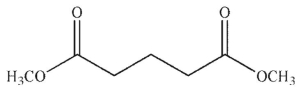
C)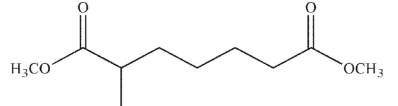
D)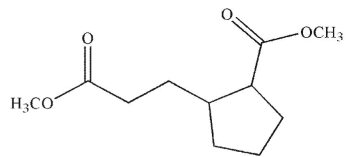
E)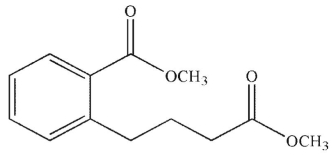
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following is a mechanistic intermediate in this Mannich reaction? 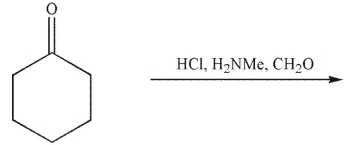
A)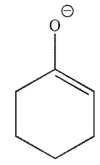
B)
C)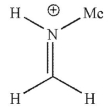
D)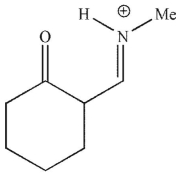
E)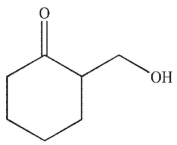

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following esters cannot undergo a successful Claisen condensation? 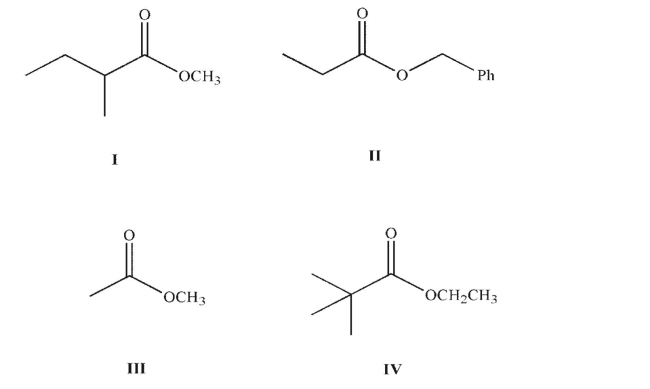
A)I
B)I and II
C)II and III
D)IV
E)I and IV

A)I
B)I and II
C)II and III
D)IV
E)I and IV

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
34
Provide a mechanism for the following transformation. 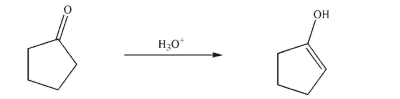


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
35
What is the product of the following reaction conditions? 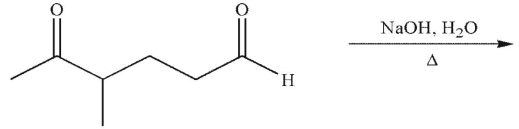
A)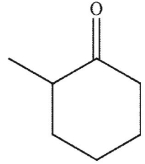
B)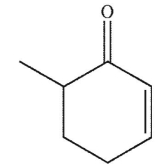
C)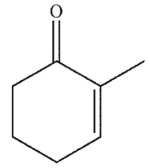
D)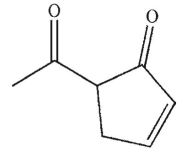
E) None of these is the product of the conditions shown.

A)

B)

C)

D)

E) None of these is the product of the conditions shown.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
36
What is the product of the reaction conditions shown? 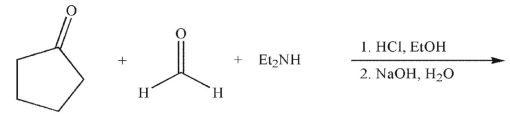
A)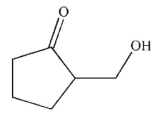
B)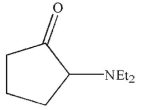
C)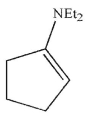
D)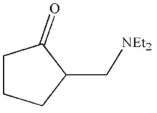
E) None of these structures is the product of the reaction conditions shown.

A)

B)

C)

D)

E) None of these structures is the product of the reaction conditions shown.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
37
Which of these compounds could reasonably form under the reaction conditions? 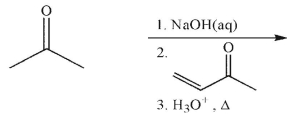
A)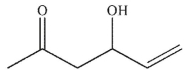
B)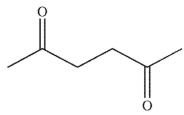
C)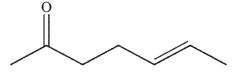
D)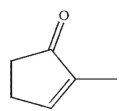
E)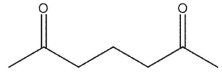
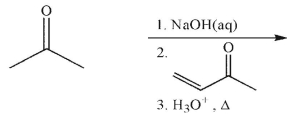
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
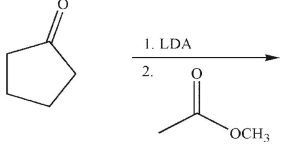
38
What is the product of this reaction?
A)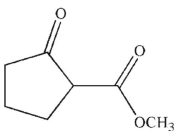
B)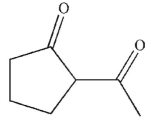
C)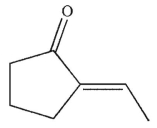
D)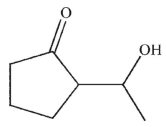
E)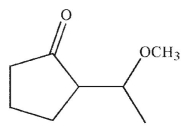

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
39
What is the product of the reaction conditions shown? 
A)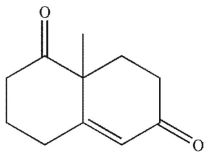
B)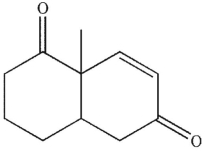
C)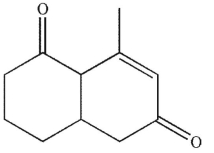
D)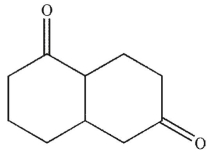
E)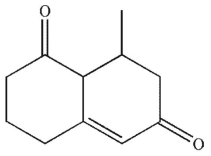

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
40
Provide a mechanism for the following transformation. 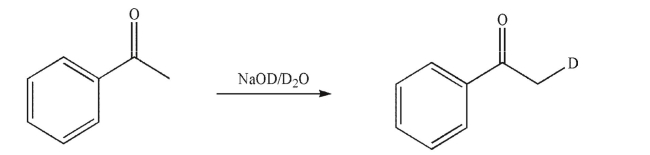


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
41
Predict the product of the reaction sequence shown. 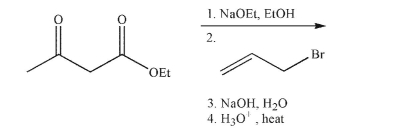


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
42
Draw a mechanism for the following transformation.Include all necessary lone pairs of electrons,
curved arrows, and nonzero formal charges.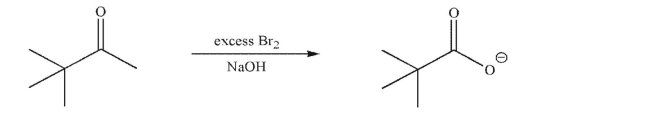
curved arrows, and nonzero formal charges.


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
43
Predict the product of the following reaction sequence and draw a mechanism to rationalize its
formation.Include all necessary lone pairs of electrons, curved arrows, and nonzero formal
charges.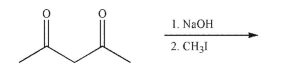
formation.Include all necessary lone pairs of electrons, curved arrows, and nonzero formal
charges.


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
44
Halogenation at the α position of carbonyls can be performed in acidic or basic conditions, but
with different results.In acidic conditions, the reaction stops at the monohalogenated product, but
under basic conditions, additional halogenations occur.Explain.
with different results.In acidic conditions, the reaction stops at the monohalogenated product, but
under basic conditions, additional halogenations occur.Explain.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
45
What is the product of the following reaction? 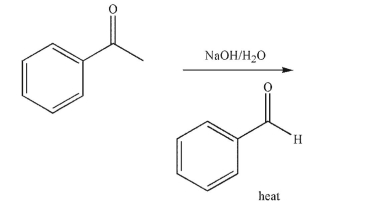


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
46
Draw the product of the following reaction. 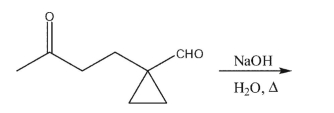


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
47
Devise a multistep synthesis for the following transformation.Show the reagents needed for each
step and the product of each step.Do not draw any mechanisms.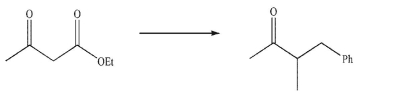
step and the product of each step.Do not draw any mechanisms.


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
48
Predict the product of the following transformation and draw a mechanism to rationalize its
formation.Include all necessary lone pairs of electrons, curved arrows, and nonzero formal
charges.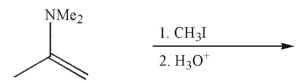
formation.Include all necessary lone pairs of electrons, curved arrows, and nonzero formal
charges.
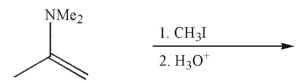

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
49
Devise a multistep synthesis for the following transformation.Show the reagents needed for each
step and the product of each step.Do not draw any mechanisms.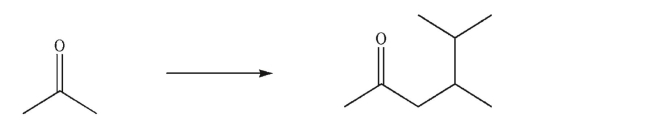
step and the product of each step.Do not draw any mechanisms.


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
50
Draw a mechanism for the following transformation.Include all necessary lone pairs of electrons,
curved arrows, and nonzero formal charges.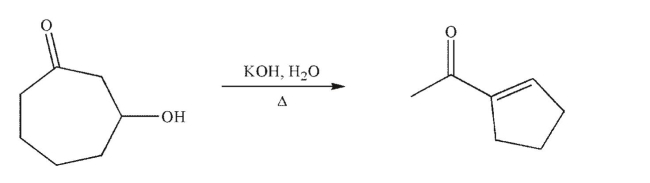
curved arrows, and nonzero formal charges.


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
51
Draw the product from the following synthesis. 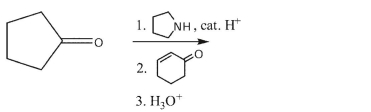


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
52
Draw a mechanism to illustrate the transformation shown here.Include all necessary lone pairs of
electrons, curved arrows, and nonzero formal charges.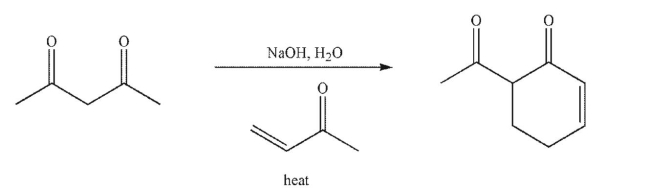
electrons, curved arrows, and nonzero formal charges.


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
53
Devise a multistep synthesis for the following transformation.Show the reagents needed for each
step and the product of each step.Do not draw any mechanisms.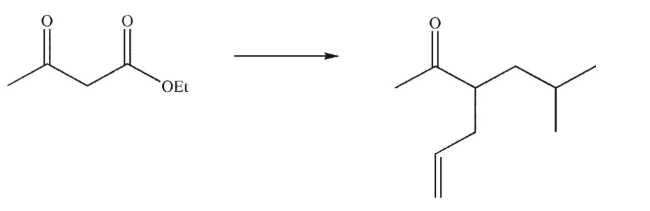
step and the product of each step.Do not draw any mechanisms.


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
54
Draw a mechanism for the transformation shown here. Include all necessary lone pairs of electrons, curved arrows, and nonzero formal charges.
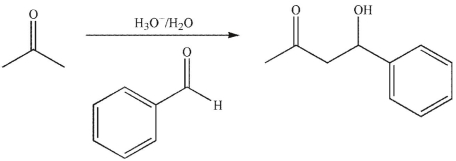


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
55
Draw a mechanism for the transformation shown here.Include any necessary lone pairs of
electrons, curved arrows, and nonzero formal charges.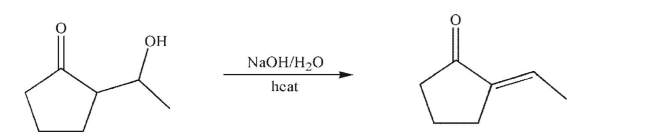
electrons, curved arrows, and nonzero formal charges.


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
56
Show the principal product(s) of the following reaction: 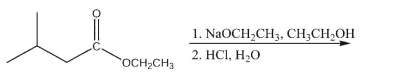
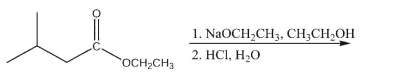

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
57
Devise a multistep synthesis for the following transformation.Show the reagents needed for each
step and the product of each step.Do not draw any mechanisms.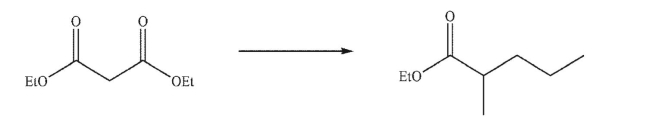
step and the product of each step.Do not draw any mechanisms.


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
58
Predict the product of the following reaction. 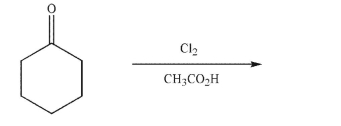


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
59
Draw the intermediates and final product in the synthetic sequence shown. 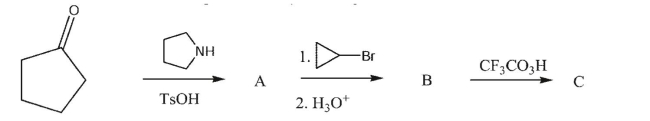


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
60
Devise a multistep synthesis for the following transformation.Show the reagents needed for each
step and the product of each step.Do not draw any mechanisms.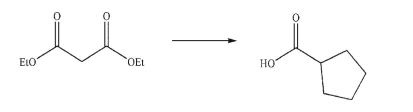
step and the product of each step.Do not draw any mechanisms.


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
61
Devise a multistep synthesis for the following transformation. Show the reagents needed for each step and the product of each step. Do not draw any mechanisms.
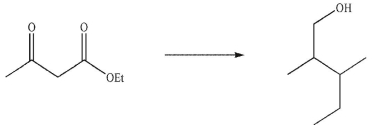


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
62
Design a multistep synthesis of the target molecule from the indicated starting material.Show the
reagents needed for each step and the product of each step.Do not show any mechanisms.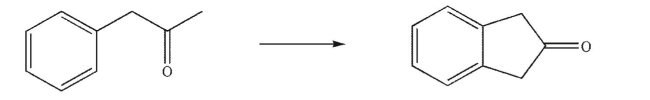
reagents needed for each step and the product of each step.Do not show any mechanisms.


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
63
Draw the product of the following reaction.
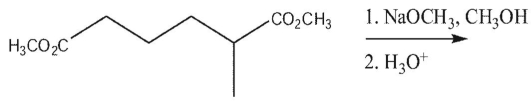


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
64
Provide the missing reagents in the following reaction sequence. 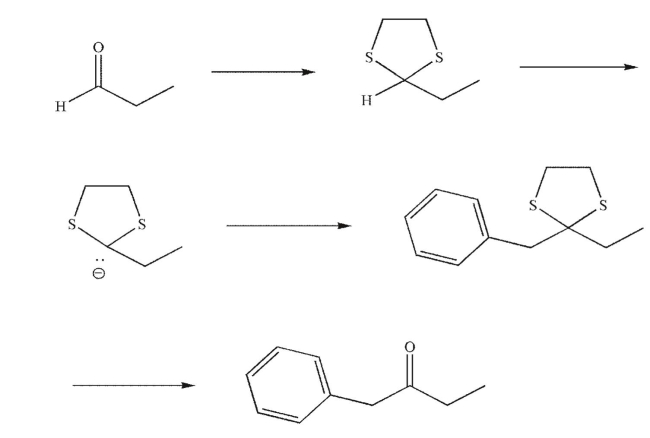


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
65
Devise a multistep synthesis for the following transformation. Show the reagents needed for each step and the product of each step. Do not draw any mechanisms.
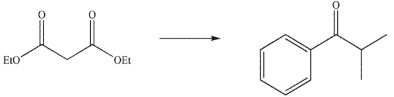


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
66
The ester shown here cannot undergo a successful Claisen condensation.Explain why. 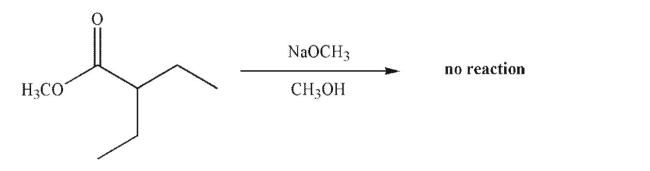


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
67
Show the mechanism of the following reaction.
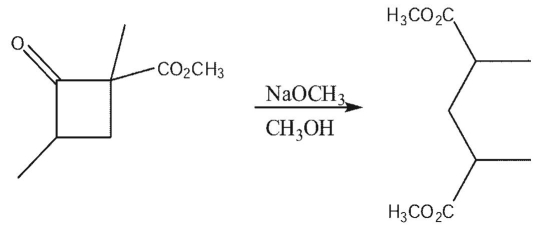


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
68
Draw the principal product in the following synthesis. 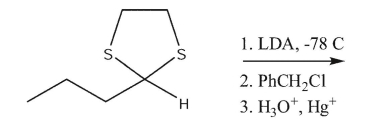


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
69
Show the mechanism of the reaction that acetone undergoes in the presence of aqueous base. 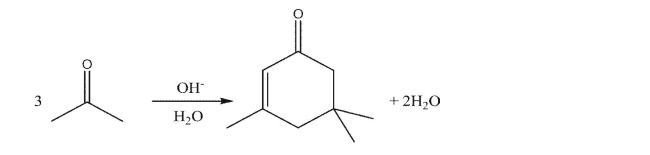


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
70
Draw the structures of the starting materials and any reagents necessary to synthesize this
compound using a Claisen condensation.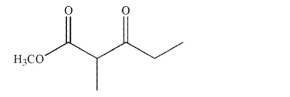
compound using a Claisen condensation.


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
71
Design a multistep synthesis of the target molecule from the indicated starting material.Show the
reagents needed for each step and the product of each step.Do not show any mechanisms.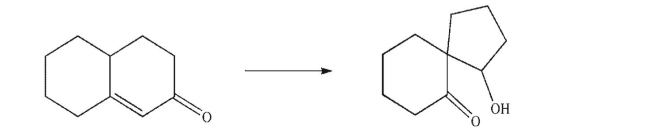
reagents needed for each step and the product of each step.Do not show any mechanisms.


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck



