Deck 15: Nuclear Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/106
Play
Full screen (f)
Deck 15: Nuclear Chemistry
1
Which of the following forms of radiation is most penetrating?
A)alpha
B)beta
C)gamma
D)positron
E)proton
A)alpha
B)beta
C)gamma
D)positron
E)proton
gamma
2
Which of the following processes results in the conversion of a neutron to a proton within a nucleus?
A)alpha particle emission
B)beta (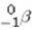 )particle emission
)particle emission
C)positron emission
D)neutron emission
E)proton emission
A)alpha particle emission
B)beta (
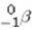 )particle emission
)particle emissionC)positron emission
D)neutron emission
E)proton emission
beta ( 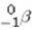 )particle emission
)particle emission
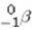 )particle emission
)particle emission 3
Rank the following in order of increasing penetrating ability: alpha particles, beta particles, gamma rays.
A)alpha particles < beta particles < gamma rays
B)alpha particles < gamma rays < beta particles
C)gamma rays < beta particles < alpha particles
D)beta particles < alpha particles < gamma rays
E)beta particles < gamma rays < alpha particles
A)alpha particles < beta particles < gamma rays
B)alpha particles < gamma rays < beta particles
C)gamma rays < beta particles < alpha particles
D)beta particles < alpha particles < gamma rays
E)beta particles < gamma rays < alpha particles
alpha particles < beta particles < gamma rays
4
Which of the following is a form of radiation composed of negatively charged particles?
A)alpha
B)beta
C)gamma
D)positron
E)proton
A)alpha
B)beta
C)gamma
D)positron
E)proton

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
5
Which process decreases the atomic number of an element by two?
A)alpha particle emission
B)beta particle emission
C)positron emission
D)gamma ray emission
E)none of these
A)alpha particle emission
B)beta particle emission
C)positron emission
D)gamma ray emission
E)none of these

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following statements regarding nuclear chemistry is correct?
A)In the lighter elements that are stable, the neutron to proton ratio is about 2.
B)Nuclides inside the band of stability are radioactive.
C)A positron is a particle that is similar to an electron, except that it has a positive charge.
D)A beta particle is a particle that has less penetrating ability than an alpha particle.
E)An alpha particle is equivalent to the nucleus of a hydrogen atom.
A)In the lighter elements that are stable, the neutron to proton ratio is about 2.
B)Nuclides inside the band of stability are radioactive.
C)A positron is a particle that is similar to an electron, except that it has a positive charge.
D)A beta particle is a particle that has less penetrating ability than an alpha particle.
E)An alpha particle is equivalent to the nucleus of a hydrogen atom.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
7
Gamma rays are
A)high-energy electromagnetic radiation.
B)H nuclei.
C)electrons.
D)He nuclei.
E)positrons.
A)high-energy electromagnetic radiation.
B)H nuclei.
C)electrons.
D)He nuclei.
E)positrons.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
8
A beta particle has a mass number of ________ and a charge of ________.
A)1, 0
B)0, 0
C)0, 1- or 1+
D)1, 1+
E)1, 1-
A)1, 0
B)0, 0
C)0, 1- or 1+
D)1, 1+
E)1, 1-

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following forms of radiation is least penetrating?
A)alpha
B)beta
C)gamma
D)positron
E)proton
A)alpha
B)beta
C)gamma
D)positron
E)proton

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
10
Alpha particles are equivalent to
A)He atoms.
B)H atoms.
C)electrons.
D)positrons
E)He nuclei.
A)He atoms.
B)H atoms.
C)electrons.
D)positrons
E)He nuclei.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
11
Beta particles are equivalent to
A)He nuclei.
B)H atoms.
C)electrons only.
D)positrons only.
E)either electrons or positrons.
A)He nuclei.
B)H atoms.
C)electrons only.
D)positrons only.
E)either electrons or positrons.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following statements regarding nuclear chemistry is correct?
A)Atoms with the same mass number but different atomic numbers are called isotopes.
B)All isotopes are radioactive.
C)Radioactive decay always results in the emission of an alpha particle.
D)Radioactive decay processes result in a change in the nucleus of the isotope undergoing reaction.
E)A nuclear particle (a proton or neutron)is called a nuclide.
A)Atoms with the same mass number but different atomic numbers are called isotopes.
B)All isotopes are radioactive.
C)Radioactive decay always results in the emission of an alpha particle.
D)Radioactive decay processes result in a change in the nucleus of the isotope undergoing reaction.
E)A nuclear particle (a proton or neutron)is called a nuclide.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
13
Which process decreases the atomic number of an element by one?
A)both beta particle emission and electron capture
B)alpha particle emission
C)beta particle emission
D)positron emission
E)gamma ray emission
A)both beta particle emission and electron capture
B)alpha particle emission
C)beta particle emission
D)positron emission
E)gamma ray emission

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following processes results in the conversion of a proton to a neutron within a nucleus?
A)alpha particle emission
B)beta (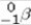 )particle emission
)particle emission
C)positron emission
D)neutron emission
E)proton emission
A)alpha particle emission
B)beta (
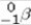 )particle emission
)particle emissionC)positron emission
D)neutron emission
E)proton emission

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following changes occur to the nucleus when a heavy element undergoes alpha particle emission?
A)Only the number of neutrons decreases.
B)Only the number of protons decreases.
C)Only the number of neutrons increases.
D)Only the number of protons increases.
E)Both the number of neutrons and protons decreases.
A)Only the number of neutrons decreases.
B)Only the number of protons decreases.
C)Only the number of neutrons increases.
D)Only the number of protons increases.
E)Both the number of neutrons and protons decreases.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
16
An alpha particle has a mass number of ________ and a charge of ________.
A)1, 2+
B)2, 0
C)4, 2+
D)4, 0
A)1, 2+
B)2, 0
C)4, 2+
D)4, 0

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following forms of radiation has no mass?
A)alpha
B)beta
C)gamma
D)positron
E)proton
A)alpha
B)beta
C)gamma
D)positron
E)proton

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
18
A gamma ray has a mass number of ________ and a charge of ________.
A)1, 0
B)1, 1+
C)-1, 1-
D)0, 0
E)0, 1+
A)1, 0
B)1, 1+
C)-1, 1-
D)0, 0
E)0, 1+

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
19
Which process increases the atomic number of an element by one?
A)gamma ray emission
B)alpha particle emission
C)beta particle emission
D)positron emission
E)electron capture
A)gamma ray emission
B)alpha particle emission
C)beta particle emission
D)positron emission
E)electron capture

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following statements regarding nuclear chemistry is correct?
A)Most of the known nuclides are stable.
B)There is no clear relationship between the stability of a radioactive nuclide and the combination of neutron and proton numbers in the nucleus.
C)A nuclide is an atom with a specific atomic number, mass number, and neutron number.
D)Nucleon is the term used to describe either a proton or an electron.
E)Each element has only one isotope.
A)Most of the known nuclides are stable.
B)There is no clear relationship between the stability of a radioactive nuclide and the combination of neutron and proton numbers in the nucleus.
C)A nuclide is an atom with a specific atomic number, mass number, and neutron number.
D)Nucleon is the term used to describe either a proton or an electron.
E)Each element has only one isotope.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
21
What is the missing symbol in the following nuclear transformation? 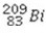 +
+ 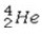 → ________ +
→ ________ + 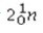
A)
B)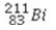
C)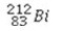
D)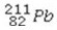
E)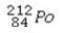
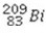 +
+ 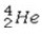 → ________ +
→ ________ + 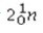
A)

B)
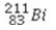
C)
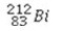
D)
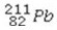
E)
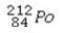

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
22
Bombardment of cobalt-59 with a neutron produces a manganese-56 atom and another particle. What is this particle?
A)an alpha particle
B)a beta particle (electron)
C)a positron
D)a gamma ray
E)a neutron
A)an alpha particle
B)a beta particle (electron)
C)a positron
D)a gamma ray
E)a neutron

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
23
Thorium-234 decays by alpha emission to form a new nuclide. Identify the new nuclide.
A)radium-228
B)radium-230
C)protactinium-234
D)protactinium-230
E)thorium-232
A)radium-228
B)radium-230
C)protactinium-234
D)protactinium-230
E)thorium-232

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
24
The first transmutation of an element was performed in 1919 by Ernest Rutherford when he bombarded nitrogen-14 with an alpha particle. The products were a proton, and another nuclide. What is the other nuclide?
A)nitrogen-18
B)nitrogen-17
C)fluorine-17
D)oxygen-17
E)oxygen-16
A)nitrogen-18
B)nitrogen-17
C)fluorine-17
D)oxygen-17
E)oxygen-16

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
25
What is the missing symbol in the following nuclear transformation? 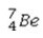 +
+ 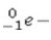 → ______
→ ______
A)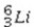
B)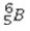
C)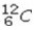
D)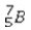
E)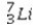
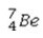 +
+ 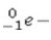 → ______
→ ______A)
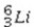
B)
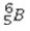
C)
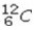
D)
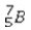
E)
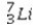

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
26
Identify the particle emitted by the nucleus that undergoes the transformation shown in the figure. 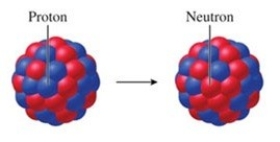
A)an alpha particle
B)a beta particle (electron)
C)a positron
D)a gamma ray
E)a neutron

A)an alpha particle
B)a beta particle (electron)
C)a positron
D)a gamma ray
E)a neutron

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
27
Bombardment of boron-10 with a projectile particle produces a nitrogen-14 atom and a gamma ray. What is the projectile particle?
A)an alpha particle
B)a beta particle (electron)
C)a positron
D)a gamma ray
E)a neutron
A)an alpha particle
B)a beta particle (electron)
C)a positron
D)a gamma ray
E)a neutron

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
28
Uranium-238 decays by alpha emission to form a new nuclide. Identify the new nuclide.
A)neptunium-238
B)protactinium-238
C)thorium-234
D)thorium-238
E)uranium-235
A)neptunium-238
B)protactinium-238
C)thorium-234
D)thorium-238
E)uranium-235

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
29
Radon-222 decays by alpha emission to form a new nuclide. Identify the new nuclide.
A)francium-222
B)francium-223
C)polonium-222
D)polonium-218
E)bismuth-220
A)francium-222
B)francium-223
C)polonium-222
D)polonium-218
E)bismuth-220

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
30
Predict the type of radiation emitted when boron-8 decays spontaneously.
A)alpha particle emission
B)beta (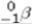 )particle emission
)particle emission
C)positron emission
D)neutron emission
E)proton emission
A)alpha particle emission
B)beta (
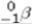 )particle emission
)particle emissionC)positron emission
D)neutron emission
E)proton emission

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
31
What is the missing symbol in the following nuclear transformation? 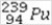 +
+ 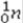 → +
→ + 
A)
B)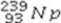
C)
D)
E)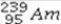
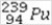 +
+ 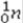 → +
→ + 
A)

B)
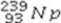
C)

D)

E)
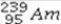

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
32
Identify the particle emitted by the nucleus that undergoes the transformation shown in the figure. 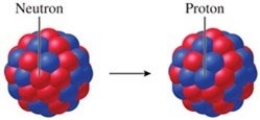
A)an alpha particle
B)a beta particle (electron)
C)a positron
D)a gamma ray
E)a neutron

A)an alpha particle
B)a beta particle (electron)
C)a positron
D)a gamma ray
E)a neutron

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
33
Bombardment of boron-10 with a neutron produces a hydrogen-1 atom and another nuclide. What is this nuclide?
A)beryllium-10
B)boron-11
C)boron-9
D)carbon-12
E)beryllium-9
A)beryllium-10
B)boron-11
C)boron-9
D)carbon-12
E)beryllium-9

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
34
Carbon-14 decays by beta emission to form a new nuclide. Identify the new nuclide.
A)nitrogen-14
B)nitrogen-13
C)carbon-12
D)carbon-13
E)beryllium-10
A)nitrogen-14
B)nitrogen-13
C)carbon-12
D)carbon-13
E)beryllium-10

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
35
Protactinium-234 decays by beta emission to form a new nuclide. Identify the new nuclide.
A)thorium-234
B)uranium-234
C)actinium-230
D)francium-232
E)thorium-233
A)thorium-234
B)uranium-234
C)actinium-230
D)francium-232
E)thorium-233

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
36
Potassium-40 decays by beta emission to form a new nuclide. Identify the new nuclide.
A)argon-40
B)chlorine-36
C)calcium-40
D)calcium-39
E)phosphorus-38
A)argon-40
B)chlorine-36
C)calcium-40
D)calcium-39
E)phosphorus-38

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
37
What is the missing symbol in the following nuclear transformation? 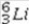 +
+ 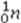 → _____ +
→ _____ + 
A)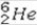
B)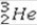
C)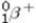
D)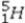
E)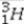
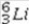 +
+ 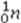 → _____ +
→ _____ + 
A)
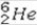
B)
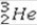
C)
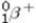
D)
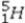
E)
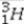

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
38
The neutron was discovered in 1932 by James Chadwick, when he bombarded beryllium-9 with an alpha particle. The products were a neutron and another nuclide. What is the other nuclide?
A)boron-12
B)lithium-8
C)carbon-13
D)carbon-12
E)boron-13
A)boron-12
B)lithium-8
C)carbon-13
D)carbon-12
E)boron-13

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
39
Thorium-234 decays by beta emission to form a new nuclide. Identify the new nuclide.
A)actinium-234
B)protactinium-231
C)protactinium-234
D)radium-230
E)radon-230
A)actinium-234
B)protactinium-231
C)protactinium-234
D)radium-230
E)radon-230

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
40
Uranium-234 decays by alpha emission to form a new nuclide. Identify the new nuclide.
A)neptunium-234
B)protactinium-234
C)thorium-230
D)thorium-234
E)uranium-235
A)neptunium-234
B)protactinium-234
C)thorium-230
D)thorium-234
E)uranium-235

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
41
Strontium-90 has a half-life of 28 years. How long will it take for 75% of the isotope to decay?
A)28 years
B)14 years
C)42 years
D)56 years
E)84 years
A)28 years
B)14 years
C)42 years
D)56 years
E)84 years

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
42
What type of radioactive decay would be expected for the unstable nuclide 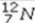 ?
?
A)alpha particle emission
B)beta particle (electron)emission
C)positron emission
D)gamma ray emission
E)neutron emission
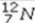 ?
?A)alpha particle emission
B)beta particle (electron)emission
C)positron emission
D)gamma ray emission
E)neutron emission

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
43
Select the most stable isotope from the following choices.
A)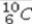
B)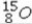
C)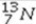
D)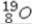
E)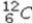
A)
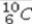
B)
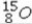
C)
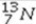
D)
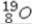
E)
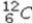

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
44
The half-life of technetium-99 is 6.0 hours. How much of a 50.0 mg sample will remain after 24 hours?
A)25.0 mg
B)12.5 mg
C)6.25 mg
D)3.12 mg
E)none of the sample will remain
A)25.0 mg
B)12.5 mg
C)6.25 mg
D)3.12 mg
E)none of the sample will remain

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
45
The half-life of technetium-99 is 6.0 hours. How much of a 25.0 mg sample will remain after 24 hours?
A)25.0 mg
B)12.5 mg
C)6.25 mg
D)3.12 mg
E)1.56 mg
A)25.0 mg
B)12.5 mg
C)6.25 mg
D)3.12 mg
E)1.56 mg

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
46
Select the most stable isotope from the following choices.
A)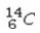
B)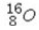
C)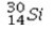
D)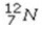
E)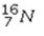
A)
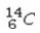
B)
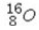
C)
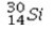
D)
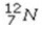
E)
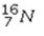

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
47
The half-life of iodine-123 is 13.3 hours. How much of a 25.0 mg sample will remain after 39.9 hours?
A)25.0 mg
B)12.5 mg
C)6.25 mg
D)3.12 mg
E)1.56 mg
A)25.0 mg
B)12.5 mg
C)6.25 mg
D)3.12 mg
E)1.56 mg

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
48
What type of radioactive decay would be expected for the unstable nuclide 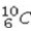 ?
?
A)alpha particle emission
B)beta particle (electron)emission
C)positron emission
D)gamma ray emission
E)neutron emission
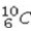 ?
?A)alpha particle emission
B)beta particle (electron)emission
C)positron emission
D)gamma ray emission
E)neutron emission

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
49
What type of radioactive decay would be expected for the unstable nuclide 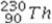 ?
?
A)alpha particle emission
B)beta particle (electron)emission
C)positron emission
D)gamma ray emission
E)neutron emission
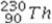 ?
?A)alpha particle emission
B)beta particle (electron)emission
C)positron emission
D)gamma ray emission
E)neutron emission

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
50
Iridium-192 has a half-life of 74 days. How long will it take for 75% of the isotope to decay?
A)74 days
B)111 days
C)37 days
D)185 days
E)148 days
A)74 days
B)111 days
C)37 days
D)185 days
E)148 days

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
51
What type of radioactive decay would be expected for the unstable nuclide 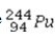 ?
?
A)alpha particle emission
B)beta particle (electron)emission
C)positron emission
D)gamma ray emission
E)neutron emission
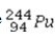 ?
?A)alpha particle emission
B)beta particle (electron)emission
C)positron emission
D)gamma ray emission
E)neutron emission

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
52
Iridium-192 has a half-life of 74 days. What fraction of a sample of this isotope will remain after 222 days?
A)1/2
B)1/4
C)1/3
D)1/8
E)1/16
A)1/2
B)1/4
C)1/3
D)1/8
E)1/16

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
53
What type of radioactive decay would be expected for the unstable nuclide 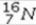 ?
?
A)alpha particle emission
B)beta particle (electron)emission
C)positron emission
D)gamma ray emission
E)neutron emission
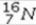 ?
?A)alpha particle emission
B)beta particle (electron)emission
C)positron emission
D)gamma ray emission
E)neutron emission

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
54
The half-life of iodine-131 is 8.1 days. How much of a 45.0 mg sample will remain after 24.3 days?
A)2.81 mg
B)5.62 mg
C)11.2 mg
D)22.5 mg
E)45.0 mg
A)2.81 mg
B)5.62 mg
C)11.2 mg
D)22.5 mg
E)45.0 mg

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
55
Predict the type of radiation that should be emitted when oxygen-18 undergoes radioactive decay.
A)beta particle (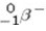 )emission
)emission
B)alpha particle emission
C)positron emission
D)neutron emission
E)proton emission
A)beta particle (
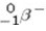 )emission
)emissionB)alpha particle emission
C)positron emission
D)neutron emission
E)proton emission

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
56
Predict the type of radiation that should be emitted when oxygen-14 undergoes radioactive decay.
A)beta particle (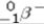 )emission
)emission
B)positron emission
C)alpha particle emission
D)proton emission
E)neutron emission
A)beta particle (
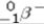 )emission
)emissionB)positron emission
C)alpha particle emission
D)proton emission
E)neutron emission

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
57
The half-life of iodine-123 is 13.3 hours. How much of a 50.0 mg sample will remain after 39.9 hours?
A)50.0 mg
B)25.0 mg
C)12.5 mg
D)6.25 mg
E)3.12 mg
A)50.0 mg
B)25.0 mg
C)12.5 mg
D)6.25 mg
E)3.12 mg

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
58
Phosphorus-32 has a half-life of 14 days. What fraction of a sample of this isotope will remain after 6 weeks?
A)1/2
B)1/4
C)1/3
D)1/8
E)1/16
A)1/2
B)1/4
C)1/3
D)1/8
E)1/16

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
59
Select the most stable isotope from the following choices.
A)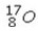
B)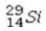
C)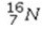
D)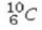
E)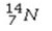
A)
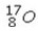
B)
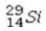
C)
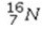
D)
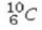
E)
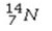

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
60
The half-life of iodine-131 is 8.1 days. How much of a 75.0 mg sample will remain after 24.3 days?
A)75.0 mg
B)37.5 mg
C)18.8 mg
D)9.38 mg
E)4.68 mg
A)75.0 mg
B)37.5 mg
C)18.8 mg
D)9.38 mg
E)4.68 mg

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following isotopes would be the best choice for use with PET?
A)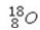
B)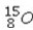
C)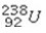
D)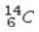
E)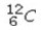
A)
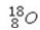
B)
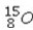
C)
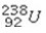
D)
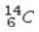
E)

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
62
The reaction  +
+ 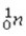 →
→ 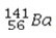 +
+ 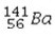 + 3
+ 3 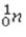 is an example of
is an example of
A)a fission reaction.
B)beta-particle emission.
C)a fusion reaction.
D)an electron capture reaction.
E)alpha-particle emission.
 +
+ 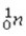 →
→ 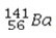 +
+ 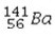 + 3
+ 3 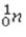 is an example of
is an example ofA)a fission reaction.
B)beta-particle emission.
C)a fusion reaction.
D)an electron capture reaction.
E)alpha-particle emission.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
63
Carbon-14 has a half-life of 5730 yr. A living organism has an activity of 15.2 counts per minute (cpm)per gram of carbon. If a bone is determined to have an activity of 3.80 cpm per gram of carbon, how old is the bone?
A)5730 yr
B)17,200 yr
C)22,900 yr
D)8600 yr
E)11,500 yr
A)5730 yr
B)17,200 yr
C)22,900 yr
D)8600 yr
E)11,500 yr

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
64
What product is formed when  undergoes 2 alpha emissions and 2 beta emissions in a total of four steps?
undergoes 2 alpha emissions and 2 beta emissions in a total of four steps?
A)actinium-227
B)thorium-227
C)thorium-231
D)polonium-231
E)radon-227
 undergoes 2 alpha emissions and 2 beta emissions in a total of four steps?
undergoes 2 alpha emissions and 2 beta emissions in a total of four steps?A)actinium-227
B)thorium-227
C)thorium-231
D)polonium-231
E)radon-227

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
65
Carbon-11 radioactively decays by positron emission with a half-life of 20.4 minutes. If a dosage of carbon-11 is administered to a patient for a PET scan, what percentage of carbon-11 will remain in the patient's system after 81.6 min?
A)75.0%
B)12.5%
C)87.5%
D)25.0%
E)6.25%
A)75.0%
B)12.5%
C)87.5%
D)25.0%
E)6.25%

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following statements regarding the possible effects of radiation on a cell is incorrect?
A)Radiation can pass through a cell with no damage.
B)Radiation can damage a cell, but it is possible for the cell to repair the damage.
C)Radiation may damage a cell so severely that it cannot repair itself.
D)A damaged cell may mutate, and cause cancer.
E)The degree of damage does not depend on the ionizing ability of the radiation.
A)Radiation can pass through a cell with no damage.
B)Radiation can damage a cell, but it is possible for the cell to repair the damage.
C)Radiation may damage a cell so severely that it cannot repair itself.
D)A damaged cell may mutate, and cause cancer.
E)The degree of damage does not depend on the ionizing ability of the radiation.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
67
Carbon-14 has a half-life of 5730 yr. A living organism has an activity of 15.2 counts per minute (cpm)per gram of carbon. If a bone is determined to have an activity of 1.90 cpm per gram of carbon, how old is the bone?
A)5730 yr
B)17,200 yr
C)22,900 yr
D)8600 yr
E)11,500 yr
A)5730 yr
B)17,200 yr
C)22,900 yr
D)8600 yr
E)11,500 yr

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
68
Carbon-11 radioactively decays by positron emission with a half-life of 20.4 minutes. If a dosage of carbon-11 is administered to a patient for a PET scan, what percentage of carbon-11 will remain in the patient's system after 61.2 min?
A)75.0%
B)12.5%
C)87.5%
D)25.0%
E)50.0%
A)75.0%
B)12.5%
C)87.5%
D)25.0%
E)50.0%

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
69
Why is radon gas dangerous?
A)It reacts chemically with lung tissue.
B)It emits gamma rays.
C)It undergoes fusion reactions, producing lots of energy.
D)It undergoes fission reactions at a very high rate.
E)Its decay products are radioactive solids that can attach to lung tissue and cause cell damage upon decay.
A)It reacts chemically with lung tissue.
B)It emits gamma rays.
C)It undergoes fusion reactions, producing lots of energy.
D)It undergoes fission reactions at a very high rate.
E)Its decay products are radioactive solids that can attach to lung tissue and cause cell damage upon decay.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
70
What product is formed when 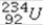 undergoes 5 alpha emissions and 2 beta emissions in a total of seven steps?
undergoes 5 alpha emissions and 2 beta emissions in a total of seven steps?
A)mercury-214
B)polonium-214
C)polonium-224
D)mercury-204
E)plutonium-239
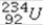 undergoes 5 alpha emissions and 2 beta emissions in a total of seven steps?
undergoes 5 alpha emissions and 2 beta emissions in a total of seven steps?A)mercury-214
B)polonium-214
C)polonium-224
D)mercury-204
E)plutonium-239

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
71
What kind of isotope is used for PET scans?
A)proton absorbers
B)neutron emitters
C)positron emitters
D)beta emitters
E)neutron absorbers
A)proton absorbers
B)neutron emitters
C)positron emitters
D)beta emitters
E)neutron absorbers

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
72
What is product of the first step of radioactive decay when  decays by alpha emission?
decays by alpha emission?
A)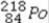
B)
C)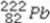
D)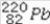
E)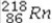
 decays by alpha emission?
decays by alpha emission?A)
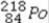
B)

C)
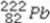
D)
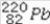
E)
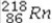

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following statements regarding nuclear stability is incorrect?
A)Isotopes with excess mass tend to undergo alpha decay.
B)Isotopes with an N/Z ratio that is too high tend to undergo beta decay.
C)Isotopes with an N/Z ratio that is too low tend to undergo either positron emission or electron capture.
D)Electron capture is the most common mode of decay for smaller isotopes.
E)An energetically excited nucleus can undergo gamma emission.
A)Isotopes with excess mass tend to undergo alpha decay.
B)Isotopes with an N/Z ratio that is too high tend to undergo beta decay.
C)Isotopes with an N/Z ratio that is too low tend to undergo either positron emission or electron capture.
D)Electron capture is the most common mode of decay for smaller isotopes.
E)An energetically excited nucleus can undergo gamma emission.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following statements is incorrect?
A)Pacemakers for heart patients are powered by a plutonium-238 power device.
B)Iodine-131 is used for diagnosing thyroid tumors since iodine is preferentially absorbed by the thyroid gland.
C)Metastable technetium-99 is used for medical diagnosis because it is absorbed by tumor cells, but not normal brain cells.
D)Carbon-14 is used for PET imaging due to its long half-life and stability.
E)Gamma radiation from cobalt-60 is used to treat cancer patients.
A)Pacemakers for heart patients are powered by a plutonium-238 power device.
B)Iodine-131 is used for diagnosing thyroid tumors since iodine is preferentially absorbed by the thyroid gland.
C)Metastable technetium-99 is used for medical diagnosis because it is absorbed by tumor cells, but not normal brain cells.
D)Carbon-14 is used for PET imaging due to its long half-life and stability.
E)Gamma radiation from cobalt-60 is used to treat cancer patients.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
75
The reaction  +
+ 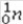 →
→ 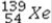 +
+ 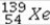 + 2
+ 2 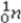 is an example of
is an example of
A)a fusion reaction.
B)beta-particle emission.
C)a fission reaction.
D)an electron capture reaction.
E)alpha-particle emission.
 +
+ 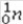 →
→ 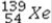 +
+ 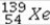 + 2
+ 2 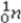 is an example of
is an example ofA)a fusion reaction.
B)beta-particle emission.
C)a fission reaction.
D)an electron capture reaction.
E)alpha-particle emission.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
76
The figure shows an example of 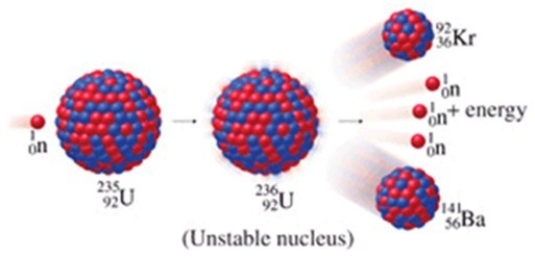
A)a fusion reaction.
B)beta-particle emission.
C)a fission reaction.
D)an electron capture reaction.
E)alpha-particle emission.

A)a fusion reaction.
B)beta-particle emission.
C)a fission reaction.
D)an electron capture reaction.
E)alpha-particle emission.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
77
What product is formed when 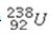 undergoes 2 alpha emissions and 2 beta emissions in a total of four steps?
undergoes 2 alpha emissions and 2 beta emissions in a total of four steps?
A)lead-234
B)radon-234
C)thorium-230
D)radon-232
E)radon-228
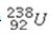 undergoes 2 alpha emissions and 2 beta emissions in a total of four steps?
undergoes 2 alpha emissions and 2 beta emissions in a total of four steps?A)lead-234
B)radon-234
C)thorium-230
D)radon-232
E)radon-228

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
78
What product is formed when 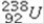 undergoes 6 alpha emissions and 3 beta emissions in a total of nine steps?
undergoes 6 alpha emissions and 3 beta emissions in a total of nine steps?
A)lead-223
B)mercury-223
C)mercury-226
D)bismuth-214
E)lead-214
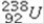 undergoes 6 alpha emissions and 3 beta emissions in a total of nine steps?
undergoes 6 alpha emissions and 3 beta emissions in a total of nine steps?A)lead-223
B)mercury-223
C)mercury-226
D)bismuth-214
E)lead-214

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following statements regarding nuclear stability is incorrect?
A)The N/Z ratio is about 1 for the first 20 elements.
B)The N/Z ratio is about 1.6 for elements at the upper end of the band of stability.
C)An N/Z ratio of 1 means that an element has an equal number of protons and electrons.
D)Elements outside the band of stability are radioactive.
E)All isotopes with Z greater than 83 are radioactive.
A)The N/Z ratio is about 1 for the first 20 elements.
B)The N/Z ratio is about 1.6 for elements at the upper end of the band of stability.
C)An N/Z ratio of 1 means that an element has an equal number of protons and electrons.
D)Elements outside the band of stability are radioactive.
E)All isotopes with Z greater than 83 are radioactive.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
80
When bombarded by a neutron, uranium-235 undergoes fission. If the nuclides produced are xenon-139 and strontium-95, how many neutrons are emitted?
A)none
B)1
C)2
D)3
E)4
A)none
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck



