Deck 21: Household Chemicals
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/162
Play
Full screen (f)
Deck 21: Household Chemicals
1
Floating soaps are
A) composed of short chain fatty acids.
B) composed of branched chain fatty acids.
C) blown with air during processing.
D) detergents.
A) composed of short chain fatty acids.
B) composed of branched chain fatty acids.
C) blown with air during processing.
D) detergents.
blown with air during processing.
2
In acidic solutions, soaps are converted to
A) salts.
B) fatty acids.
C) detergents.
D) fats.
A) salts.
B) fatty acids.
C) detergents.
D) fats.
fatty acids.
3
Which of the following is NOT an advantage of soap? Soap is
A) biodegradable.
B) derived from renewable resources.
C) an excellent cleaner in hard water.
D) relatively nontoxic.
A) biodegradable.
B) derived from renewable resources.
C) an excellent cleaner in hard water.
D) relatively nontoxic.
an excellent cleaner in hard water.
4
The majority of household chemicals are
A) cleaning agents.
B) laundry products.
C) cosmetics.
D) paints.
A) cleaning agents.
B) laundry products.
C) cosmetics.
D) paints.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
5
Animal fats and vegetable oil can be converted to soaps by reaction with
A) sodium hydroxide.
B) sodium carbonate.
C) sodium hyperchlorite.
D) sodium phosphate.
A) sodium hydroxide.
B) sodium carbonate.
C) sodium hyperchlorite.
D) sodium phosphate.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
6
American pioneers combined potash solution and animal fat in a huge iron kettle and cooked it over several hours. The result was
A) detergent.
B) soap.
C) cooking oil.
D) food for animals.
A) detergent.
B) soap.
C) cooking oil.
D) food for animals.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
7
Sodium carbonate (Na2CO3) and potassium carbonate (K2CO3) react with water to form an alkaline solution that has detergent properties. These compounds are present in
A) detergents.
B) lye based soaps.
C) saponins.
D) plant ashes.
A) detergents.
B) lye based soaps.
C) saponins.
D) plant ashes.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
8
Soap is
A) a glycerol ester.
B) a salt of a fatty acid.
C) lithium sulfate.
D) glycerol.
A) a glycerol ester.
B) a salt of a fatty acid.
C) lithium sulfate.
D) glycerol.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
9
Water softeners remove all of the following ions EXCEPT
A) Ca2+.
B) Fe2+.
C) Mg2+.
D) Na+.
A) Ca2+.
B) Fe2+.
C) Mg2+.
D) Na+.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
10
Soap can be made by boiling animal fat with lye or potash for several hours. What is the major disadvantage to the lye soap made this way?
A) It did not clean well.
B) It often contained unreacted alkali, which was very harsh on the skin.
C) Glycerol separated out from the soap and remained on the bottom of the kettle.
D) It did not kill bacteria.
A) It did not clean well.
B) It often contained unreacted alkali, which was very harsh on the skin.
C) Glycerol separated out from the soap and remained on the bottom of the kettle.
D) It did not kill bacteria.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
11
Washing soda is
A) sodium peroxide.
B) sodium carbonate.
C) sodium borate.
D) sodium chloride.
A) sodium peroxide.
B) sodium carbonate.
C) sodium borate.
D) sodium chloride.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
12
The substance with the formula shown below is a(n) CH3CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2COOH
A) alcohol.
B) detergent.
C) saponin.
D) fatty acid.
A) alcohol.
B) detergent.
C) saponin.
D) fatty acid.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
13
The molecule shown below is a(n) 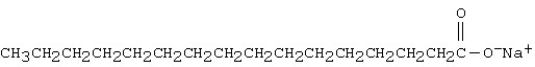
A) detergent.
B) saponin.
C) soap.
D) esterester.
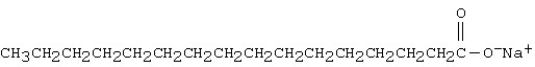
A) detergent.
B) saponin.
C) soap.
D) esterester.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
14
In cleaning, soap acts as a(n) ________ between "dirt" and water.
A) catalyst
B) chemical reactant
C) emulsifier
D) insulator
A) catalyst
B) chemical reactant
C) emulsifier
D) insulator

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
15
Bathtub ring is caused by
A) the action of ammonia with water.
B) precipitation of soap by "hard" metal ions.
C) rust formation from sulfur in the water.
D) soap and water cause a ringing sound in the ears.
A) the action of ammonia with water.
B) precipitation of soap by "hard" metal ions.
C) rust formation from sulfur in the water.
D) soap and water cause a ringing sound in the ears.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
16
One of the major problems with the use of household chemicals is
A) they are toxic.
B) consumers often fail to read directions and warnings.
C) they do not perform well.
D) all of the above
A) they are toxic.
B) consumers often fail to read directions and warnings.
C) they do not perform well.
D) all of the above

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
17
In hard water, soaps are converted to
A) insoluble salts.
B) bases.
C) lye.
D) anhydrides.
A) insoluble salts.
B) bases.
C) lye.
D) anhydrides.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
18
An advantage of potassium soaps is that they are ________ than sodium soaps.
A) softer
B) harder
C) more neutral
D) stronger
A) softer
B) harder
C) more neutral
D) stronger

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
19
Water softeners
A) remove "hard" ions.
B) destroy "hard" ions.
C) modify "hard" ions.
D) Water softeners do all of the above.
A) remove "hard" ions.
B) destroy "hard" ions.
C) modify "hard" ions.
D) Water softeners do all of the above.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following statements about soaps and detergents is NOT true?
A) The hydrophilic end has the hydrocarbon chain.
B) The hydrophilic end is ionic.
C) The hydrophobic end has the hydrocarbon chain.
D) Soaps form micelles in solution.
A) The hydrophilic end has the hydrocarbon chain.
B) The hydrophilic end is ionic.
C) The hydrophobic end has the hydrocarbon chain.
D) Soaps form micelles in solution.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
21
The active ingredient in chlorine laundry bleaches is
A) chlorine, Cl2.
B) sodium hypochlorite, NaOCl.
C) sodium chloride, NaCl.
D) a mixture of CFCs.
A) chlorine, Cl2.
B) sodium hypochlorite, NaOCl.
C) sodium chloride, NaCl.
D) a mixture of CFCs.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
22
The major advantage of LAS detergents over ABS detergents is that they
A) are effective in hard water.
B) are soil based.
C) are biodegradable.
D) lack phosphates.
A) are effective in hard water.
B) are soil based.
C) are biodegradable.
D) lack phosphates.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
23
Soaps are
A) anionic surfactants.
B) nonionic surfactants.
C) neutral surfactants.
D) positive surfactants.
A) anionic surfactants.
B) nonionic surfactants.
C) neutral surfactants.
D) positive surfactants.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
24
Detergents are better cleaners than soaps in
A) hard water.
B) soft water.
C) alkaline water.
D) all of the above
A) hard water.
B) soft water.
C) alkaline water.
D) all of the above

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
25
Sequestration is
A) precipitating magnesium and calcium ions.
B) when sodium and lithium ions are tied up in soluble complexes.
C) when calcium and magnesium ions are tied up in soluble complexes.
D) a nonionic surfactant.
A) precipitating magnesium and calcium ions.
B) when sodium and lithium ions are tied up in soluble complexes.
C) when calcium and magnesium ions are tied up in soluble complexes.
D) a nonionic surfactant.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
26
Automatic dishwashing detergents are
A) similar to detergents for hand washing.
B) relatively strong bases.
C) relatively strong acids.
D) safe for hand washing of dishes.
A) similar to detergents for hand washing.
B) relatively strong bases.
C) relatively strong acids.
D) safe for hand washing of dishes.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
27
Substances added to surfactants to increase their detergency are
A) brighteners.
B) builders.
C) emulsifiers.
D) enzymes.
A) brighteners.
B) builders.
C) emulsifiers.
D) enzymes.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
28
Which water softener "ties up" calcium ions and keeps them in solution?
A) sodium carbonate
B) sodium phosphate
C) fatty acids
D) zeolites
A) sodium carbonate
B) sodium phosphate
C) fatty acids
D) zeolites

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following is NOT a component of detergents for washing dishes by hand?
A) enzymes
B) fragrances
C) strong alkalis
D) surfactants
A) enzymes
B) fragrances
C) strong alkalis
D) surfactants

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
30
Optical brighteners are often added to
A) detergents.
B) soaps.
C) cosmetics.
D) They are added to all of the above.
A) detergents.
B) soaps.
C) cosmetics.
D) They are added to all of the above.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following categories of surfactants gives the least amount of sudsing?
A) amphoteric surfactants
B) anionic surfactants
C) cationic surfactants
D) nonionic surfactants
A) amphoteric surfactants
B) anionic surfactants
C) cationic surfactants
D) nonionic surfactants

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
32
Quaternary salts with two long carbon chains and two smaller chains on the nitrogen are used as
A) bleaches.
B) analgesics.
C) fabric softeners.
D) weed killers.
A) bleaches.
B) analgesics.
C) fabric softeners.
D) weed killers.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
33
Cationic surfactants are not good detergents but are used mainly for their ________ action.
A) cleaning
B) menthol
C) germicidal
D) darkening
A) cleaning
B) menthol
C) germicidal
D) darkening

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
34
The active ingredient in oxygen bleaches is
A) NaOCl.
B) NaBO2 ∙ H2O2.
C) O2.
D) Na3PO4.
A) NaOCl.
B) NaBO2 ∙ H2O2.
C) O2.
D) Na3PO4.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
35
Optical brighteners work by
A) absorbing blue rays and ultraviolet rays.
B) absorbing ultraviolet rays and emitting blue rays.
C) spontaneously emitting blue rays when they attach to clothing fibers.
D) spontaneously emitting ultraviolet rays when they attach to clothing fibers.
A) absorbing blue rays and ultraviolet rays.
B) absorbing ultraviolet rays and emitting blue rays.
C) spontaneously emitting blue rays when they attach to clothing fibers.
D) spontaneously emitting ultraviolet rays when they attach to clothing fibers.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
36
The molecule shown below is a 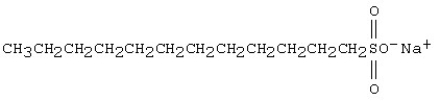
A) soap.
B) detergent.
C) fatty acid.
D) polymer.
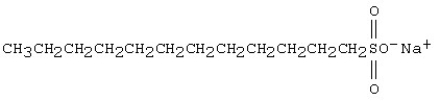
A) soap.
B) detergent.
C) fatty acid.
D) polymer.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
37
Why are perborate bleaches better for white, resin-treated, polyester-cotton fabrics than chlorine bleaches?
A) Chlorine bleaches release a toxic gas when in contact with this type of fabric.
B) Chlorine bleaches ruin the fabric.
C) Oxygen bleaches are cheaper.
D) The fabric lasts longer.
A) Chlorine bleaches release a toxic gas when in contact with this type of fabric.
B) Chlorine bleaches ruin the fabric.
C) Oxygen bleaches are cheaper.
D) The fabric lasts longer.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following statements is NOT true for fabric softeners?
A) They are anionic surfactants.
B) They are quaternary salts.
C) They form a film which lubricates the fibers in the fabric.
D) They have two long hydrocarbon chains.
A) They are anionic surfactants.
B) They are quaternary salts.
C) They form a film which lubricates the fibers in the fabric.
D) They have two long hydrocarbon chains.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
39
A problem with ABS detergents is that they
A) are toxic.
B) do not degrade readily.
C) contain phosphates.
D) are less effective in hard water.
A) are toxic.
B) do not degrade readily.
C) contain phosphates.
D) are less effective in hard water.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following forms a strongly caustic solution when mixed with water?
A) automatic dishwashing detergent
B) liquid dishwashing detergent
C) liquid clothing detergent
D) bath soap
A) automatic dishwashing detergent
B) liquid dishwashing detergent
C) liquid clothing detergent
D) bath soap

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
41
All of the following are used as binders for paint EXCEPT
A) acrylic resins.
B) linseed oil.
C) polyvinyl acetate.
D) All of the above are binders.
A) acrylic resins.
B) linseed oil.
C) polyvinyl acetate.
D) All of the above are binders.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
42
What type of chemical is most effective in removing the build-up of "lime" that is often found in toilet bowls?
A) an abrasive
B) an acid
C) a base
D) a bleach
A) an abrasive
B) an acid
C) a base
D) a bleach

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
43
A wax is a(n)
A) ether made from two long-chain alcohols.
B) ester made from a long-chain fatty acid and a long-chain alcohol.
C) long-chain compound that contains both an alcohol group and an amino group.
D) amide made from a long-chain fatty acid and a long-chain amine.
A) ether made from two long-chain alcohols.
B) ester made from a long-chain fatty acid and a long-chain alcohol.
C) long-chain compound that contains both an alcohol group and an amino group.
D) amide made from a long-chain fatty acid and a long-chain amine.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
44
A cleaning product that may contain either ammonia or vinegar in a dilute solution of isopropyl (rubbing) alcohol would be used as a(n)
A) toilet bowl cleaner.
B) oven cleaner.
C) glass cleaner.
D) drain cleaner.
A) toilet bowl cleaner.
B) oven cleaner.
C) glass cleaner.
D) drain cleaner.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
45
A green product would most likely NOT contain which of the following?
A) a natural scent
B) materials derived from plant sources
C) degradable ingredients
D) volatile hydrocarbons
A) a natural scent
B) materials derived from plant sources
C) degradable ingredients
D) volatile hydrocarbons

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
46
Which one of the following is NOT considered a cosmetic?
A) soap
B) lipstick
C) toothpaste
D) facial cream
A) soap
B) lipstick
C) toothpaste
D) facial cream

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
47
The main ingredient in oven cleaners is
A) acetic acid (vinegar).
B) bleach.
C) citric acid.
D) sodium hydroxide.
A) acetic acid (vinegar).
B) bleach.
C) citric acid.
D) sodium hydroxide.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
48
Fabric softeners are
A) potassium salts of long chain fatty acids.
B) oxidizing agents.
C) quaternary salts with two long carbon chains and two smaller chains on the nitrogen.
D) nonionic surfactants.
A) potassium salts of long chain fatty acids.
B) oxidizing agents.
C) quaternary salts with two long carbon chains and two smaller chains on the nitrogen.
D) nonionic surfactants.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
49
Baking soda can be used as
A) an automatic dishwashing detergent.
B) a germicide.
C) a substance to absorb odor.
D) an acid.
A) an automatic dishwashing detergent.
B) a germicide.
C) a substance to absorb odor.
D) an acid.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following ingredients will NOT be found in paint?
A) white lead
B) titanium dioxide
C) a solvent
D) a binder
A) white lead
B) titanium dioxide
C) a solvent
D) a binder

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
51
A molecule that contains only C, H and O atoms will degrade most quickly if its structure
A) is a straight chain.
B) contains many branches.
C) contains rings.
D) also has halogen atoms.
A) is a straight chain.
B) contains many branches.
C) contains rings.
D) also has halogen atoms.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
52
Bleaches work by
A) changing the structure of color-producing groups called chromophores to make them colorless.
B) acting directly on the soiled spot to remove the soil.
C) changing the stains so that they absorb UV light and then release blue light.
D) covering the stains with quaternary ammonium ions so that they are no longer visible.
A) changing the structure of color-producing groups called chromophores to make them colorless.
B) acting directly on the soiled spot to remove the soil.
C) changing the stains so that they absorb UV light and then release blue light.
D) covering the stains with quaternary ammonium ions so that they are no longer visible.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
53
Bleaches are
A) oxidizing agents.
B) reducing agents.
C) brighteners.
D) detergents.
A) oxidizing agents.
B) reducing agents.
C) brighteners.
D) detergents.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
54
The abrasive in commercial powdered cleansers is often
A) sodium carbonate.
B) baking powder.
C) charcoal.
D) gold metal.
A) sodium carbonate.
B) baking powder.
C) charcoal.
D) gold metal.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following is a hazard of using organic solvents in the home? Organic solvents are
A) flammable.
B) toxic.
C) volatile.
D) All of the above are hazards.
A) flammable.
B) toxic.
C) volatile.
D) All of the above are hazards.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
56
Household ammonia solutions are
A) acidic.
B) basic.
C) neutral.
D) oxidizing agents.
A) acidic.
B) basic.
C) neutral.
D) oxidizing agents.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
57
When we buy products for household use, we should avoid products whose packaging is
A) reusable.
B) recyclable.
C) degradable.
D) longest lasting.
A) reusable.
B) recyclable.
C) degradable.
D) longest lasting.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
58
Lanolin comes from
A) beeswax.
B) palm tree leaves.
C) sheep's wool.
D) sea urchins.
A) beeswax.
B) palm tree leaves.
C) sheep's wool.
D) sea urchins.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following ingredients is NOT found in drain cleaners?
A) aluminum filings
B) bleach
C) HCl
D) NaOH
A) aluminum filings
B) bleach
C) HCl
D) NaOH

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following is no longer used as a component of paint?
A) polymers
B) 2PbCO3 ∙ Pb(OH)2
C) tung oil
D) ester
A) polymers
B) 2PbCO3 ∙ Pb(OH)2
C) tung oil
D) ester

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
61
A cosmetic that is a suspension of oil in water is called a
A) wax.
B) lotion.
C) lanolin.
D) cream.
A) wax.
B) lotion.
C) lanolin.
D) cream.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
62
Deodorants act by
A) breaking down odorous chemicals as they are produced.
B) destroying odor-causing bacteria.
C) producing enzymes.
D) reacting with sweat glands to stop perspiration.
A) breaking down odorous chemicals as they are produced.
B) destroying odor-causing bacteria.
C) producing enzymes.
D) reacting with sweat glands to stop perspiration.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
63
Astringents are used primarily in
A) perfumes.
B) lipsticks.
C) antiperspirants.
D) hair spray.
A) perfumes.
B) lipsticks.
C) antiperspirants.
D) hair spray.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
64
Powdered eye shadows have ________ as a base.
A) talc
B) sugar
C) corn starch
D) peanut oil
A) talc
B) sugar
C) corn starch
D) peanut oil

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
65
Emollients are
A) artificial skin.
B) skin plasticizers.
C) skin coatings.
D) skin catalysts.
A) artificial skin.
B) skin plasticizers.
C) skin coatings.
D) skin catalysts.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
66
Which generally does NOT need to be proven safe and effective before marketing?
A) cosmetic
B) drug
C) food additive
D) a cooking oil
A) cosmetic
B) drug
C) food additive
D) a cooking oil

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
67
White eye shadow is "colored" with titanium dioxide or
A) lampblack.
B) chromic oxide.
C) lead sulfide.
D) zinc oxide.
A) lampblack.
B) chromic oxide.
C) lead sulfide.
D) zinc oxide.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
68
Which is NOT a cosmetic?
A) antidandruff shampoo
B) lipstick
C) blush
D) perfume
A) antidandruff shampoo
B) lipstick
C) blush
D) perfume

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
69
The oil secreted by glands in the skin is called
A) sweat.
B) sebum.
C) melanin.
D) musk.
A) sweat.
B) sebum.
C) melanin.
D) musk.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
70
Skin moisturizers
A) add moisture to skin.
B) prevent loss of moisture from skin.
C) cause skin to produce more water.
D) do all of the above.
A) add moisture to skin.
B) prevent loss of moisture from skin.
C) cause skin to produce more water.
D) do all of the above.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
71
The ideal moisture content of skin is approximately
A) 3%.
B) 10%.
C) 16%.
D) 75%.
A) 3%.
B) 10%.
C) 16%.
D) 75%.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
72
A cosmetic that is a suspension of water in oil is called a
A) cream.
B) sunscreen.
C) moisturizer.
D) wax.
A) cream.
B) sunscreen.
C) moisturizer.
D) wax.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
73
The layer of skin that contains sweat glands and hair follicles is the
A) apocrine.
B) dermis.
C) epidermis.
D) eccrine.
A) apocrine.
B) dermis.
C) epidermis.
D) eccrine.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
74
A major problem with eye makeups is
A) bacterial contamination after extended use.
B) color fading.
C) allergic reactions.
D) all of the above
A) bacterial contamination after extended use.
B) color fading.
C) allergic reactions.
D) all of the above

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
75
The outer layer of skin is called the
A) exoskin.
B) episkin.
C) epidermis.
D) exodermis.
A) exoskin.
B) episkin.
C) epidermis.
D) exodermis.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
76
A typical ingredient in skin lotions and creams is
A) palm oil.
B) pork fat.
C) corn oil.
D) walnut oil.
A) palm oil.
B) pork fat.
C) corn oil.
D) walnut oil.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
77
The active ingredient in almost all antiperspirants is
A) aluminum chlorohydrate.
B) ethanol.
C) ethylene oxide.
D) aluminum oxide.
A) aluminum chlorohydrate.
B) ethanol.
C) ethylene oxide.
D) aluminum oxide.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
78
Skin cream and lipsticks have approximately the same basic composition. What is added to lipsticks to make them more firm?
A) wax
B) cellulose
C) petroleum jelly
D) abrasive
A) wax
B) cellulose
C) petroleum jelly
D) abrasive

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
79
Exposure of skin to UV radiation does NOT cause
A) melanin production.
B) increased risk of skin cancer.
C) premature aging of skin.
D) a reverse in the aging process.
A) melanin production.
B) increased risk of skin cancer.
C) premature aging of skin.
D) a reverse in the aging process.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
80
Sunscreens contain chemicals that
A) absorb both UV-A and UV-B rays.
B) absorb neither UV-A nor UV-B rays.
C) inhibit melanin production in the skin.
D) promote melanin production in skin.
A) absorb both UV-A and UV-B rays.
B) absorb neither UV-A nor UV-B rays.
C) inhibit melanin production in the skin.
D) promote melanin production in skin.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck



