Deck 13: Chemical Equilibrium and Poisons
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/20
Play
Full screen (f)
Deck 13: Chemical Equilibrium and Poisons
1
Which classification contains substances that decompose the protein molecules that make up your body?
A) carcinogen
B) corrosive
C) mutagen
D) teratogen
A) carcinogen
B) corrosive
C) mutagen
D) teratogen
corrosive
2
Which of the following refers to a naturally occurring poisonous substance produced by a living organism?
A) mutagen
B) poison
C) teratogen
D) toxin
A) mutagen
B) poison
C) teratogen
D) toxin
toxin
3
How does the presence of a catalyst affect equilibrium?
A) It decreases the amount of time it takes for the reaction to reach equilibrium.
B) It increases the amount of time it takes for the reaction to reach equilibrium.
C) It does not change the amount of time it takes for the reaction to reach equilibrium.
A) It decreases the amount of time it takes for the reaction to reach equilibrium.
B) It increases the amount of time it takes for the reaction to reach equilibrium.
C) It does not change the amount of time it takes for the reaction to reach equilibrium.
It decreases the amount of time it takes for the reaction to reach equilibrium.
4
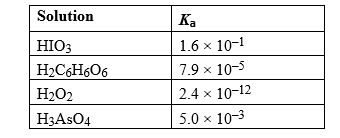
Given the following list of Ka values, determine which weak acid solution would have the lowest pH value at equilibrium assuming all acids have the same initial concentration.
A) HIO3
B) H2C6H6O6
C) H2O2
D) H3AsO4

A) HIO3
B) H2C6H6O6
C) H2O2
D) H3AsO4

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
5
Determine the equilibrium concentration of bromide when the lead concentration is 2.8 × 10-3 M. The Ksp of PbBr2 is 6.60 × 10-6.
A) 0.0024 M
B) 0.049 M
C) 1.2 M
D) 424 M
A) 0.0024 M
B) 0.049 M
C) 1.2 M
D) 424 M

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following refers to a substance that alters the DNA of cells in the parent, which can cause disease in offspring?
A) carcinogen
B) mutagen
C) poison
D) teratogen
A) carcinogen
B) mutagen
C) poison
D) teratogen

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
7
Determine the equilibrium concentration of cadmium when the carbonate concentration is 5.4 × 10-7 M. The Ksp for CdCO3 is 1.0 × 10-12.
A) 5.4 × 10-19 M
B) 1.9 × 10-6 M
C) 5.4 × 105 M
D) 6.8 × 108 M
A) 5.4 × 10-19 M
B) 1.9 × 10-6 M
C) 5.4 × 105 M
D) 6.8 × 108 M

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following describes how a system at equilibrium will respond to a decrease in product concentration?
A) The equilibrium will shift to the left.
B) The equilibrium will shift to the right.
C) The equilibrium will not shift to either the right or the left.
A) The equilibrium will shift to the left.
B) The equilibrium will shift to the right.
C) The equilibrium will not shift to either the right or the left.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
9
Given the following Ksp values, determine which solution has the greatest concentration of ions at equilibrium.
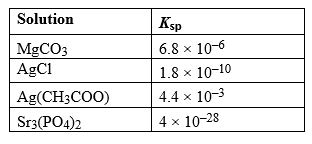
A) MgCO3
B) AgCl
C) Ag(CH3COO)
D) Sr3(PO4)2

A) MgCO3
B) AgCl
C) Ag(CH3COO)
D) Sr3(PO4)2

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
10
All the reactants are completely changed to products in chemical reactions.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
11
Which conditions are present when K is very large?
A) low concentrations of both products and reactants
B) high concentrations of both products and reactants
C) high concentration of reactants and low concentration of products
D) high concentration of products and low concentration of reactants
A) low concentrations of both products and reactants
B) high concentrations of both products and reactants
C) high concentration of reactants and low concentration of products
D) high concentration of products and low concentration of reactants

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
12
Is the LD50 a measurement for acute poisoning or chronic poisoning?
A) acute
B) chronic
A) acute
B) chronic

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following describes how a system at equilibrium will respond to an increase in reactant concentration?
A) The equilibrium will shift to the left.
B) The equilibrium will shift to the right.
C) The equilibrium will not shift to either the right or the left.
A) The equilibrium will shift to the left.
B) The equilibrium will shift to the right.
C) The equilibrium will not shift to either the right or the left.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
14
Determine the solubility equilibrium constant for CaF2 given that the equilibrium concentration of [Ca2+] = 0.00039 M and [F-] = 0.00032 M. Ksp = ______________

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
15
Which statement is true for a reaction at dynamic equilibrium?
A) The temperature is constant.
B) The forward and reverse reactions are both occurring.
C) The concentrations of the products and reactants are equal.
D) The concentrations of the products and reactants are constant.
A) The temperature is constant.
B) The forward and reverse reactions are both occurring.
C) The concentrations of the products and reactants are equal.
D) The concentrations of the products and reactants are constant.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
16
Which statement describes when dynamic equilibrium occurs?
A) Only the reverse reaction occurs.
B) Only the forward reaction occurs.
C) The rate of the forward and reverse reactions is equal.
D) The rate of the forward and the reverse reactions is zero.
A) Only the reverse reaction occurs.
B) Only the forward reaction occurs.
C) The rate of the forward and reverse reactions is equal.
D) The rate of the forward and the reverse reactions is zero.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
17
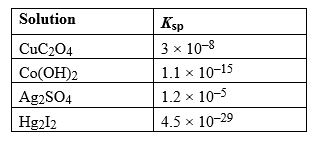
Given the following Ksp values, determine which solution has the smallest concentration of ions at equilibrium.
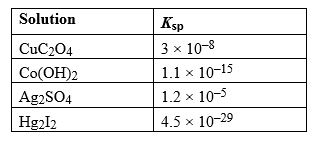
A) CuC2O4
B) Co(OH)2
C) Ag2SO4
D) Hg2I2

A) CuC2O4
B) Co(OH)2
C) Ag2SO4
D) Hg2I2

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
18
Given the following list of Ka values, determine which weak acid solution would have the highest pH value at equilibrium assuming all acids have the same initial concentration.
A) HC2O4-
B) HSO4-
C) H2PO4-
D) H2AsO4-

A) HC2O4-
B) HSO4-
C) H2PO4-
D) H2AsO4-

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
19
Carbon monoxide poisoning occurs when hemoglobin binds to the carbon monoxide as shown:
HbO2 + CO ⇌ HbCO + O2
Explain giving excess oxygen to a patient with mild carbon monoxide poisoning helps them.
HbO2 + CO ⇌ HbCO + O2
Explain giving excess oxygen to a patient with mild carbon monoxide poisoning helps them.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
20
The equilibrium constant for a reaction is 0.045. Which of the following is true for this reaction?
A) Equilibrium lies to the left.
B) Equilibrium lies to the right.
C) The reaction favors the product.
A) Equilibrium lies to the left.
B) Equilibrium lies to the right.
C) The reaction favors the product.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck



