Deck 11: Heat
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/99
Play
Full screen (f)
Deck 11: Heat
1
A typical person's normal metabolic rate (the rate at which food/stored energy is consumed) is about 4 × 10 5 J/h, and the average food energy in a Big Mac is 600 Calories. If a person lived on nothing but Big Macs, how many per day would he or she have to eat to maintain a constant body weight
Thinking It Through:
Metabolic rate of normal person,
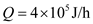 That is the rate at which it converts food to stored energy in heat, movement and so on.
That is the rate at which it converts food to stored energy in heat, movement and so on.
Average food energy in a Big Mac, E
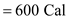 By using the units conversion,
By using the units conversion,
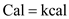
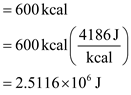 The numbers of Big Macs have to eat per day to maintain the weight is given as by dividing the metabolic rate of normal person per day with average food energy of Big Mac.
The numbers of Big Macs have to eat per day to maintain the weight is given as by dividing the metabolic rate of normal person per day with average food energy of Big Mac.
Solution:
Metabolic rate of normal person per hour
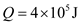 Metabolic rate of normal person per day
Metabolic rate of normal person per day
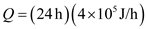 Therefore the number of Big Macs eaten to maintain a constant body weight is
Therefore the number of Big Macs eaten to maintain a constant body weight is
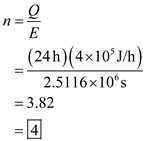
Metabolic rate of normal person,
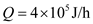 That is the rate at which it converts food to stored energy in heat, movement and so on.
That is the rate at which it converts food to stored energy in heat, movement and so on. Average food energy in a Big Mac, E
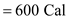 By using the units conversion,
By using the units conversion,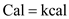
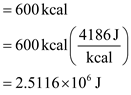 The numbers of Big Macs have to eat per day to maintain the weight is given as by dividing the metabolic rate of normal person per day with average food energy of Big Mac.
The numbers of Big Macs have to eat per day to maintain the weight is given as by dividing the metabolic rate of normal person per day with average food energy of Big Mac.Solution:
Metabolic rate of normal person per hour
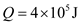 Metabolic rate of normal person per day
Metabolic rate of normal person per day 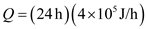 Therefore the number of Big Macs eaten to maintain a constant body weight is
Therefore the number of Big Macs eaten to maintain a constant body weight is 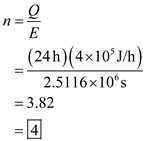
2
Latent heat is always (a) part of the specific heat, (b) related to the specific heat, (c) the same as the mechanical equivalent of heat, (d) none of the preceding.
Specific heat: The amount of heat required to increase the temperature of a body by
 raise in temperature.
raise in temperature.
Latent heat: The amount of heat used to change the state of matter, without changing its temperature.
Mechanical equivalent of heat: The amount of work required to increase the temperature of a unit mass by
 Both Specific heat and mechanical equivalent of heat involve change in temperature. But, the latent heat involves change in state but not temperature. Therefore, the correct option is (d).
Both Specific heat and mechanical equivalent of heat involve change in temperature. But, the latent heat involves change in state but not temperature. Therefore, the correct option is (d).
 raise in temperature.
raise in temperature.Latent heat: The amount of heat used to change the state of matter, without changing its temperature.
Mechanical equivalent of heat: The amount of work required to increase the temperature of a unit mass by
 Both Specific heat and mechanical equivalent of heat involve change in temperature. But, the latent heat involves change in state but not temperature. Therefore, the correct option is (d).
Both Specific heat and mechanical equivalent of heat involve change in temperature. But, the latent heat involves change in state but not temperature. Therefore, the correct option is (d). 3
A 0.250-kg coffee cup at 20 °C is filled with 0.250 kg of brewed coffee at 100 °C. Tire cup and the coffee come to thermal equilibrium at 80 °C. If no heat is lost to the environment, what is the specific heat of the cup material [ Hint : Consider the coffee essentially to be water.]
A coffee cup is filled with coffee. There is no heat loss to the environment, so the total energy of the system is conserved.
The amount of heat required to change the temperature of the substance is,
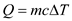 Here, m is mass of the substance, c is specific heat of the substance, and
Here, m is mass of the substance, c is specific heat of the substance, and
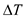 is change in temperature.
is change in temperature.
Since there is no heat loss to the environment, the heat loss by coffee is equal to the heat gained by the cup.
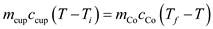 Here,
Here,
 is mass of cup,
is mass of cup,
 is specific heat of cup,
is specific heat of cup,
 is temperature of cup,
is temperature of cup,
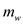 is mass of water,
is mass of water,
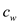 is the specific heat of water,
is the specific heat of water,
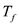 is temperature of water, and T is equilibrium temperature.
is temperature of water, and T is equilibrium temperature.
Substitute 0.250 kg for
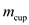 , 0.250 kg for
, 0.250 kg for
 ,
,
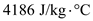 for
for
 ,
,
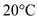 for
for
 ,
,
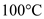 for
for
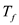 , and
, and
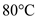 for T.
for T.
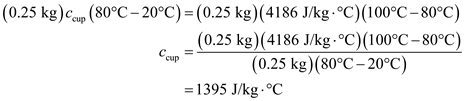 Therefore, the specific heat of cup is
Therefore, the specific heat of cup is
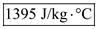 .
.
The amount of heat required to change the temperature of the substance is,
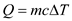 Here, m is mass of the substance, c is specific heat of the substance, and
Here, m is mass of the substance, c is specific heat of the substance, and 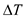 is change in temperature.
is change in temperature.Since there is no heat loss to the environment, the heat loss by coffee is equal to the heat gained by the cup.
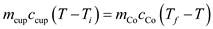 Here,
Here,  is mass of cup,
is mass of cup,  is specific heat of cup,
is specific heat of cup, is temperature of cup,
is temperature of cup, 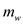 is mass of water,
is mass of water,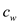 is the specific heat of water,
is the specific heat of water,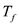 is temperature of water, and T is equilibrium temperature.
is temperature of water, and T is equilibrium temperature.Substitute 0.250 kg for
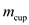 , 0.250 kg for
, 0.250 kg for ,
, 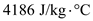 for
for  ,
, 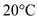 for
for  ,
,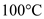 for
for 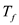 , and
, and 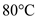 for T.
for T. 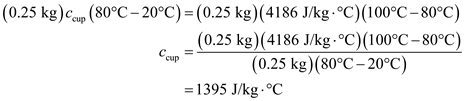 Therefore, the specific heat of cup is
Therefore, the specific heat of cup is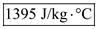 .
. 4
How much heat is required to completely boil away 0.50 L of liquid nitrogen at 196 °C (Take the density of liquid nitrogen to be 0.80 × 10 3 kg/m 3.)

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
5
Assume that your skin has an emissivity of 0.70, a normal temperature of 34 °C, and a total exposed area of 0.25 m 2. How much heat energy per second do you lose due to radiation if the outside temperature is 22 °C

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
6
A steel cylinder of radius 5.0 cm and length 4.0 cm is placed in end-to-end thermal contact with a copper cylinder of the same dimensions. If the free ends of the two cylinders are maintained at constant temperatures of 95 °C (steel) and 15 °C (copper), how much heat will flow through the cylinders in 20 min

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
7
The amount of heat necessary to change the temperature of 1 kg of a substance by 1°C is called the substance's (a) specific heat, (b) latent heat, (c) heat of combustion, (d) mechanical equivalent of heat.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
8
In general, you would get a more severe burn from steam at 100 °C than from the same mass of hot water at 100 °C. Why

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
9
An aluminum spoon at 100 °C is placed in a Styrofoam cup containing 0.200 kg of water at 20 °C. If the final equilibrium temperature is 30 °C and no heat is lost to the cup itself or the environment, what is the mass of the aluminum spoon

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
10
An alcohol rub can rapidly decrease body (skin) temperature. (a) This is because of (1) the cooler temperature of the alcohol, (2) the evaporation of alcohol, (3) the high specific heat of the human body. (b) To decrease the body temperature of a 65-kg person by 1.0 °C, what mass of alcohol must be evaporated from the person's skin Ignore the heat involved in raising the temperature of alcohol to its boiling point (why ) and approximate the human body as water.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
11
The U.S. five-cent coin, the nickel, has a mass of 5.1 g, a volume of 0.719 cm 3 , and a total surface area of 8.54 cm 2. Assuming that a nickel is an ideal radiator, how much radiant energy per second comes from the nickel, if it is at 20 °C

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
12
In Exercise, what is the temperature at the interface of the cylinders
Exercise
A steel cylinder of radius 5.0 cm and length 4.0 cm is placed in end-to-end thermal contact with a copper cylinder of the same dimensions. If the free ends of the two cylinders are maintained at constant temperatures of 95 °C (steel) and 15 °C (copper), how much heat will flow through the cylinders in 20 min
Exercise
A steel cylinder of radius 5.0 cm and length 4.0 cm is placed in end-to-end thermal contact with a copper cylinder of the same dimensions. If the free ends of the two cylinders are maintained at constant temperatures of 95 °C (steel) and 15 °C (copper), how much heat will flow through the cylinders in 20 min

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
13
Equal amounts of heat are added to two different objects at the same initial temperature. What factors can cause the final temperature of the two objects to be different

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
14
Equal amounts of heat are added to an aluminum block and a copper block of different masses to achieve the same temperature increase. (a) The mass of the aluminum block is (1) more, (2) the same, (3) less than the mass of the copper block. Why (b) If the mass of the copper block is 3.00 kg, what is the mass of the aluminum block

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
15
A student doing an experiment pours 0.150 kg of heated copper shot into a 0.375-kg aluminum calorimeter cup containing 0.200 kg of water. The cup and water are both initially at 25 °C. The mixture (and the cup) comes to thermal equilibrium at 28 °C. What was the initial temperature of the shot

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
16
Heat has to be removed to condense mercury vapor at a temperature of 630 K into liquid mercury. (a) This heat involves (1) only specific heat, (2) only latent heat, or (3) both specific and latent heats. Explain. (b) If the mass of the mercury vapor is 15 g, how much heat would have to be removed

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
17
An aluminum bar and a copper bar of identical cross-sectional area have the same temperature difference between their ends and conduct heat at the same rate. (a) The copper bar is (1) longer, (2) of the same length, (3) shorter than the aluminum bar. Why (b) Calculate the ratio of the length of the copper bar to that of the aluminum bar.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
18
A 0.60 kg piece of ice at 14 °F is placed in 0.30 kg of water at 323 K. How much liquid is left when the system reaches thermal equilibrium

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
19
A student ate a Thanksgiving dinner that totaled 2800 Cal. He wants to use up all that energy by lifting a 20-kg mass a distance of 1.0 m. Assume that he lifts the mass with constant velocity and no work is required in lowering the mass. (a) How many times must he lift the mass (b) If he can lift and lower the mass once every 5.0 s, how long does this exercise take

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
20
When a substance undergoes a phase change, the added heat changes (a) the temperature, (b) the kinetic energy, (c) the potential energy, (d) the mass of the substance.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
21
At what average rate would heat have to be removed from 1.5 L of (a) water and (b) mercury to reduce the liquid's temperature from 20 °C to its freezing point in 3.0 min

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
22
If 0.050 kg of ice at 0 °C is added to 0.300 kg of water at 25 °C in a 0.100-kg aluminum calorimeter cup, what is the final temperature of the water

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
23
A copper teakettle has a circular bottom 30.0 cm in diameter that has a uniform thickness of 2.50 mm. It sits on a burner whose temperature is 150 °C. (a) If the teakettle is full of boiling water, what is the rate of heat conduction through its bottom (b) Assuming that the heat from the burner is the only heat input, how much water is boiled away in 5.0 min Is your answer unreasonably large If yes, explain why.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
24
A large Styrofoam cooler has a surface area of 1.0 m 2 and a thickness of 2.5 cm. If 5.0 kg of ice at 0 °C is stored inside and the outside temperature is a constant 35 °C, how long does it take for all the ice to melt (Consider conduction only.)

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
25
The same amount of heat Q is added to two objects of the same mass. If object 1 experienced a greater temperature change than object 2, that is, T 1 T 2 , then (a) c 1 c 2 , (b) c 1 c 2 , (c) c 1 = c 2.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
26
When you breathe out in the winter, you can see your breath, like fog. Explain.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
27
When resting, a person gives off heat at a rate of about 100 W. If the person is submerged in a tub containing 150 kg of water at 27 °C and the heat from the person goes only into the water, how many hours will it take for the water temperature to rise to 28 °C

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
28
How much ice (at 0 °C) must be added to 0.500 kg of water at 100 °C in a 0.200-kg aluminum calorimeter cup to end up with all liquid at 20 °C

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
29
Assuming that the human body has a 1.0-cm-thick layer of skin tissue and a surface area of 1.5 m 2 , estimate the rate at which heat is conducted from inside the body to the surface if the skin temperature is 34 °C. (Assume a normal body temperature of.37 °C for the temperature of the interior.)

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
30
A 1600-kg automobile traveling at 55 mph brakes smoothly to a stop. Assume 40% of the heat generated in stopping the car is dissipated in the front steel brake disks. Each front disk has a mass of 3.0 kg. What is the temperature rise of the front brake disks during the stop

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
31
Many people have performed fire walking, in which a bed of red-hot coals (temperature over 2000 °F) is walked on with bare feet. (You should not try this at home!) How is this possible [ Hint : Human tissues largely consist of water.]

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
32
A modern engine of alloy construction consists of 25 kg of aluminum and 80 kg of iron. How much heat does the engine absorb as its temperature increases from 20 °C to 100 °C as it warms up to operating temperature

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
33
To determine the specific heat of a new metal alloy, 0.150 kg of the substance is heated to 400 °C and then placed in a 0.200-kg aluminum calorimeter cup containing 0.400 kg of water at 10.0 °C. If the final temperature of the mixture is 30.5 °C, what is the specific heat of the alloy (Ignore the calorimeter stirrer and thermometer.)

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
34
Ice (initially at 0 °C) is added to 0.75 L of tea at 20 °C to make the coldest possible iced tea. If enough ice is added so the final mixture is all liquid, how much liquid is in the pitcher when this condition occurs

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
35
The emissivity of an object is 0.50. (a) Compared with a perfect blackbody at /he same temperature, this object would radiate (1) more power, (2) the same amount of power, (3) less power. Why (b) Calculate the ratio of the power radiated by the blackbody to that radiated by the object.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
36
A waterfall is 75 m high. If 20% of the gravitational potential energy of the water went into heating the water, by how much would the temperature of the water, increase in going horn the top of the falls to the bottom [ Hint : Consider a kilogram of water going over the falls.]

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
37
Discuss the difference between a calorie and a Calorie.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
38
It takes 2.0 × 10 6 J of heat to bring a quantity of water from 20 °C to a boil. What is the mass of water

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
39
House insulation materials should have (a) high thermal conductivity, (b) low thermal conductivity, (c) high emissivity, (d) low emissivity.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
40
In a calorimetry experiment, 0.50 kg of a metal at 100 °C is added to 0.50 kg of water at 20 °C in an aluminum calorimeter cup. The cup has a mass of 0.250 kg. (a) if some water splashed out of the cup when the metal was added, the measured specific heat will appear to be (1) higher, (2) the same, (3) lower than the value calculated for the case in which the water does not splash out. Why (b) If the final temperature of the mixture is 25 °C, and no water splashed out, what is the specific heat of the metal

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
41
To cool a very hot piece of 4.00-kg steel at 900 °C, the steel is put into a 5.00-kg water bath at 20 °C. What is the final temperature of the steel-water mixture

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
42
A lamp filament radiates energy at a rate of 100 W when the temperature of the surroundings is 20 °C, and only 99.5 W when the surroundings are at 30 °C. If the temperature of the filament is the same in each case, what is its temperature in Celsius

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
43
A 0.030-kg lead bullet hits a steel plate, both initially at 20 °C. The bullet melts and splatters on impact. (This action has been photographed.) Assuming that 80% of the bullet's kinetic energy goes into increasing its temperature and then melting it, what is the minimum speed it must have to melt on impact

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
44
A window air conditioner has a rating of 20 000 Btu/h. What is this rating in watts

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
45
The fundamental physical principle for calorimetry is (a) Newton's second law, (b) conservation of momentum, (c) conservation of energy, (d) equilibrium.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
46
A plastic ice cube tray and a metal ice cube tray are removed from the same freezer, at the same initial temperature. However, the metal one feels cooler to the touch. Why

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
47
Lead pellets of total mass 0.60 kg are heated to 100 °C and then placed in a well-insulated aluminum cup of mass 0.20 kg that contains 0.50 kg of water initially at 17.3 °C. What is the equilibrium temperature of the mixture

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
48
Steam at 100 °C is bubbled into 0.250 kg of water at 20 °C in a calorimeter cup, where it condenses into liquid form. How much steam will have been added when the water in the cup reaches 60 °C (Ignore the effect of the cup.)

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
49
(a) If the Kelvin temperature of an object is doubled, its radiated power increases by (1) 2, (2) 4, (3) 8, (4) 16 times. Explain. (b) If its temperature is increased from 20 °C to 40 °C, by how much does the radiated power change

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
50
A cyclist with a total skin area of 1.5 m 2 is riding a bicycle on a day when the air temperature is 20 °C and her skin temperature is 34 °C. The cyclist does work at about 200 W (moving the pedals) but her efficiency is only about 20% in terms of converting energy into mechanical work. Estimate the amount of water this cyclist must evaporate per hour (through perspiration) to get rid of the excess body heat she produces. Assume a skin emissivity of 0.70.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
51
The SI unit of heat energy is the (a) calorie, (b) kilocalorie, (c) Btu, (d) joule.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
52
A hot steel ball is dropped into a cold aluminum cup containing some water. (Assume the system is an isolated.) If the ball loses 400 J of heat, what can be said according to calorimetry

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
53
Equal amounts of heat are added to different quantities of copper and lead. The temperature of the copper increases by 5.0 °C and the temperature of the lead by 10 °C. (a) The lead has (1) a greater mass than the copper, (2) the same amount of mass as the copper, (3) less mass than the copper. (b) Calculate the mass ratio of the lead to the copper to prove your answer to part (a).

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
54
A student mixes 1.0 L of water at 40 °C with 1.0 L of ethyl alcohol at 20 °C. Assuming that no heat is lost to the container or the surroundings, what is the final temperature of the mixture [ Hint : See Table 11.1.]

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
55
Evaporation of water from our skin is a very important mechanism for controlling body temperature. (a) This is because (1) water has a high specific heat, (2) water has a high latent heat of vaporization, (3) water contains more heat when hot, (4) water is a good heat conductor. (b) In a 3.5-h intense cycling race, a cyclist can loses 7.0 kg of water through perspiration. Estimate how much heat the cyclist loses in the process.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
56
A certain object with a surface temperature of 100°C is radiating heat at a rate of 200 J/s. To double the object's rate of radiation energy, what should be its surface temperature in Celsius

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
57
A 200-kg cast iron machine part at 500 °C is left to cool at room temperature. Assume the machine part is a cube and has an emissivity of 0.780. At what rate is the machine part initially losing heat due to radiation [ Hint : The density of iron can be found in Table 9.2.]

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
58
What is the main difference between internal energy and heat

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
59
The temperature of a lead block and a copper block, both 1.0 kg and at 20 °C, is to be raised to 100 °C. (a) The copper will require (1) more heat, (2) the same heat, (3) less heat than the lead. Why (b) Calculate the difference between the heat required for the two blocks to prove your answer to part (a).

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following is the dominant heat transfer mechanism by which the Earth receives energy from the Sun: (a) conduction, (b) convection, (c) radiation, or (d) all of the preceding

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
61
We all have had the experience that a room full of people always feels warmer than when the room is empty. Ten people are in a 4.0 m × 6.0 m × 3.0 m room at 20 °C. If each person gives off heat at a rate of about 100 W and there is no heat loss to the outside of the room, what is the temperature of the room after 10 min At 20 °C, the density of air is 1.2 kg/m 3 and its specific heat at constant pressure is 1005 J/(kg · °C).

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
62
A 0.400-kg piece of ice at 10 °C is placed in an equal mass of water at 30 °C. (a) When thermal equilibrium is reached between the two, (1) all the ice will melt, (2) some of the ice will melt, (3) none of the ice will melt. (b) How much ice melts

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
63
The thermal insulation used in building is commonly rated in terms of its R-value , defined as d/k , where d is the thickness of the insulation in inches and k is its thermal conductivity. (See Insight 11.2 on p. 403.) In the United States, R-values are expressed in British units. For example, 3.0 in. of foam plastic would have an R-value of 3.0/0.30 = 10, where k = 0.30 Btu · in./(ft 2 · h · °F). This value is expressed as R-10. (a) Better insulation has a (1) high, (2) low, or (3) zero R-value. Explain. (b) What thicknesses of (1) styrofoam and (2) brick would give an R-value of R-10

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
64
A person goes on a 1500-Cal-per-day diet to lose weight. What is his daily energy allowance expressed in joules

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
65
For gases, which of the following is true about the specific heat under constant pressure, c p , and specific heat under constant volume, c v : (a) c p c v , (b) c p = c v , or (c) c p c v

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
66
Why is the warning shown on the highway road sign in Fig. necessary

FIGURE A cold warning See Conceptual Question.

FIGURE A cold warning See Conceptual Question.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
67
How much heat is required to melt a 2.5-kg block of ice at 0 °C

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
68
One kilogram of a substance experimentally shows the T -versus- Q graph in Fig. (a) What are its melting and boiling points In SI units, what are (b) the specific heats of the substance during its various phases and (c) the latent heats of the substance at the various phase changes
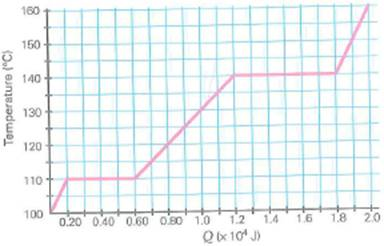
FIGURE Temperature versus heat input See Exercise.

FIGURE Temperature versus heat input See Exercise.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
69
A piece of pine 14 in. thick has an R-value of 19. For glass wool to have the same R-value, its thickness should be (a) thicker than, (2) the same as, (3) thinner than 14 in. Why (b) Calculate the required thickness of such a piece of glass wool. (See Exercise and Insight 11.2.)
Exercise
The thermal insulation used in building is commonly rated in terms of its R-value , defined as d/k , where d is the thickness of the insulation in inches and k is its thermal conductivity. (See Insight 11.2 on p. 403.) In the United States, R-values are expressed in British units. For example, 3.0 in. of foam plastic would have an R-value of 3.0/0.30 = 10, where k = 0.30 Btu · in./(ft 2 · h · °F). This value is expressed as R-10. (a) Better insulation has a (1) high, (2) low, or (3) zero R-value. Explain. (b) What thicknesses of (1) styrofoam and (2) brick would give an R-value of R-10
Exercise
The thermal insulation used in building is commonly rated in terms of its R-value , defined as d/k , where d is the thickness of the insulation in inches and k is its thermal conductivity. (See Insight 11.2 on p. 403.) In the United States, R-values are expressed in British units. For example, 3.0 in. of foam plastic would have an R-value of 3.0/0.30 = 10, where k = 0.30 Btu · in./(ft 2 · h · °F). This value is expressed as R-10. (a) Better insulation has a (1) high, (2) low, or (3) zero R-value. Explain. (b) What thicknesses of (1) styrofoam and (2) brick would give an R-value of R-10

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following is the largest unit of heat energy: (a) calorie, (b) Btu, (c) joule, or (d) kilojoule

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
71
You are monitoring the temperature of some cold ice cubes ( 5.0 °C) in a cup as the ice and cup are heated. Initially, the temperature rises, but it stops at 0 °C. After a while, it begins rising again. Is anything wrong with the thermometer Explain.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
72
Initially at 20 °C, 0.50 kg of aluminum and 0.50 kg of iron are heated to 100 °C. (a) The aluminum gains (1) more heat than the iron, (2) the same amount of heat as the iron, (3) less heat than the iron. Why (b) Calculate the difference in heat required to prove your answer to part (a).

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
73
How much heat is required to boil away 1.50 kg of water that is initially at 100 °C

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
74
In an experiment, a 0.150-kg piece of a ceramic material at 20 °C is placed in liquid nitrogen at its boiling point to cool in a perfectly insulated flask, which allows the gaseous N 2 to immediately escape. Flow many liters of liquid nitrogen will be boiled away during this operation (Take the specific heat of the ceramic material to be that of glass and the density of liquid nitrogen to be 0.80 × 10 3 kg/m 3.)

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
75
Solar heating takes advantage of solar collectors such as the type shown in Fig. During daylight hours, the average intensity of solar radiation at the top of the atmosphere is about 1400 W/m 2. About 50°/) of this radiation reaches the Earth during daylight hours. (The rest is reflected, scattered, absorbed, and so on.) How much heat energy would be received, on average, by the cylindrical collector shown in the figure during 10 h of daylight
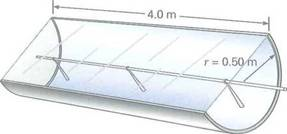
FIGURE Solar collector and solar heating See Exercise.

FIGURE Solar collector and solar heating See Exercise.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
76
If someone says that a hot object contains more heat than a cold one, would you agree Why

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
77
A 5.00-g pellet of aluminum reaches a final temperature of 63 °C when gaining 200 J of heat. What is its initial temperature

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
78
Water is a poor heat conductor, but a pot of water can be heated more quickly than you might think. This fast heating time is mainly due to heat (a) conduction, (b) convection, (c) radiation, (d) all of the preceding.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
79
(a) Converting 1.0 kg of water at 100 °C to steam at 100 °C requires (1) more heat, (2) the same amount of heat, (3) less heat than converting 1.0 kg of ice at 0 °C to water at 0 °C. Explain. (b) Calculate the difference in heat required to prove your answer to part (a).

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
80
The single glass pane in a window has dimensions of 2.00 m by 1.50 m and is 4.00 mm thick. How much heat will flow through the glass in 1.00 h if there is a temperature difference of 2 °C between the inner and outer surfaces (Consider conduction only.)

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck



