Deck 12: Thermodynamics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/118
Play
Full screen (f)
Deck 12: Thermodynamics
1
A quantity of ideal gas goes through an isothermal process and does 400 J of net work. (a) The internal energy of the gas is (1) higher than, (2) the same as, (3) less than when it started. Why (b) Is a net amount of heat added to or removed from the system, and how much is involved
a)
Isothermal process is one in which the temperature remains constant throughout the process. Assuming internal energy is a function of temperature only, we can say that there will be no change in the internal energy of system during an isothermal process. In this process the internal energy remains constant, but there will be change in pressure and volume.
Therefore, the correct option is (2).
b)
The first law of thermodynamics is essentially the principle of conservation of energy, which states that in all transformations, the total heat energy
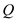 supplied must be balanced by external work done
supplied must be balanced by external work done
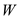 plus the increase in internal energy
plus the increase in internal energy
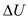 of the system and expressed as
of the system and expressed as
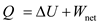 In isothermal process, the change in internal energy of the system is zero, that is
In isothermal process, the change in internal energy of the system is zero, that is
 . The mathematical form of First law of thermodynamics is
. The mathematical form of First law of thermodynamics is
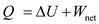 Substitute zero for
Substitute zero for
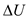 , and
, and
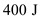 for
for
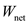 .
.
 According to sign convention, heat absorbed by the system is taken as positive, and heat given out by the system is taken as negative. Since the value of
According to sign convention, heat absorbed by the system is taken as positive, and heat given out by the system is taken as negative. Since the value of
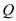 is positive, the net amount of heat will be added to the system. Hence, the amount of heat added to the system is
is positive, the net amount of heat will be added to the system. Hence, the amount of heat added to the system is
 .
.
Isothermal process is one in which the temperature remains constant throughout the process. Assuming internal energy is a function of temperature only, we can say that there will be no change in the internal energy of system during an isothermal process. In this process the internal energy remains constant, but there will be change in pressure and volume.
Therefore, the correct option is (2).
b)
The first law of thermodynamics is essentially the principle of conservation of energy, which states that in all transformations, the total heat energy
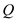 supplied must be balanced by external work done
supplied must be balanced by external work done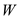 plus the increase in internal energy
plus the increase in internal energy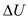 of the system and expressed as
of the system and expressed as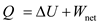 In isothermal process, the change in internal energy of the system is zero, that is
In isothermal process, the change in internal energy of the system is zero, that is . The mathematical form of First law of thermodynamics is
. The mathematical form of First law of thermodynamics is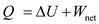 Substitute zero for
Substitute zero for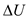 , and
, and 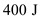 for
for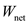 .
. According to sign convention, heat absorbed by the system is taken as positive, and heat given out by the system is taken as negative. Since the value of
According to sign convention, heat absorbed by the system is taken as positive, and heat given out by the system is taken as negative. Since the value of 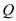 is positive, the net amount of heat will be added to the system. Hence, the amount of heat added to the system is
is positive, the net amount of heat will be added to the system. Hence, the amount of heat added to the system is .
. 2
In any natural process, the overall change in the entropy of the universe could not be (a) negative, (b) zero, (c) positive.
Entropy is a measure of the degree of disorder within the system. The entropy of the system increases as the disorder of the system increase. The entropy change is negative with a decrease in temperature and it is positive when the temperature increases.
From the Second Law of Thermodynamics,
When the process is reversible, the total entropy of the system plus its surrounding is unchanged, that is,
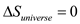 Therefore change in entropy of the universe may be zero.
Therefore change in entropy of the universe may be zero.
Hence option
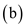 is incorrect.
is incorrect.
When the process is irreversible, the total entropy of the system plus its surrounding increases, that is,
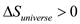 Hence option
Hence option
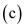 is incorrect.
is incorrect.
The universe tends towards randomness or irregularities and thus increases its temperature. Thus its entropy increases and overall change in entropy always remains positive. It cannot be negative.
Therefore, the correct option is
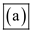 .
.
From the Second Law of Thermodynamics,
When the process is reversible, the total entropy of the system plus its surrounding is unchanged, that is,
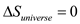 Therefore change in entropy of the universe may be zero.
Therefore change in entropy of the universe may be zero.Hence option
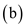 is incorrect.
is incorrect.When the process is irreversible, the total entropy of the system plus its surrounding increases, that is,
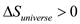 Hence option
Hence option 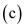 is incorrect.
is incorrect.The universe tends towards randomness or irregularities and thus increases its temperature. Thus its entropy increases and overall change in entropy always remains positive. It cannot be negative.
Therefore, the correct option is
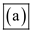 .
. 3
A student challenges the second law of thermodynamics by saying that entropy does not have to increase in all situations, such as when water freezes to ice. Is this challenge valid Why or why not
Second law of thermodynamics: In a thermal cycle, heat energy cannot be completely transformed into mechanical work.
Heat is converted to mechanical energy in many applications. In this processes, total heat is not converted to mechanical work. There must be an unusual energy. This unusual energy is responsible for increase in entropy.
No, this is not a valid challenge.
The ice and water themselves are not isolated systems. Let us consider two cases.
Case1: When water freezes into ice, it gives off heat to surroundings. In this case, there must be an unusual energy that causes change in entropy.
Case2: When ice melts into water, it takes heat from surroundings. In this case also unusual energy must present. The unusual energy causes change in entropy.
The usual heat given off by ice in case 1 is not sufficient to convert the water to ice. That means there is unusual energy that causes change in entropy.
Heat is converted to mechanical energy in many applications. In this processes, total heat is not converted to mechanical work. There must be an unusual energy. This unusual energy is responsible for increase in entropy.
No, this is not a valid challenge.
The ice and water themselves are not isolated systems. Let us consider two cases.
Case1: When water freezes into ice, it gives off heat to surroundings. In this case, there must be an unusual energy that causes change in entropy.
Case2: When ice melts into water, it takes heat from surroundings. In this case also unusual energy must present. The unusual energy causes change in entropy.
The usual heat given off by ice in case 1 is not sufficient to convert the water to ice. That means there is unusual energy that causes change in entropy.
4
What is the change in entropy of mercury vapor ( L v = 2.7 × 10 5 J/kg) when 0.50 kg of it condenses to a liquid at its boiling point of 357 °C

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
5
A system goes from state 1 to state 3 as shown on the T-S diagram in Fig. (a) The heat transfer for the process going from state 2 to state 3 is (1) positive, (2) zero, (3) negative. Explain. (b) Calculate the total heat transferred in going from state 1 to state 3.
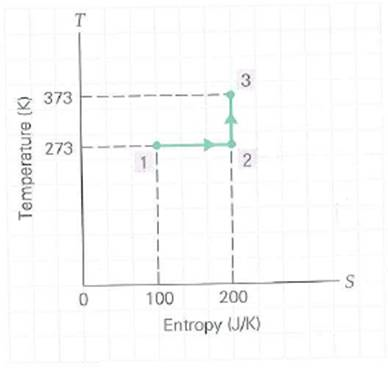
FIGURE Entropy and heat See Exercises.

FIGURE Entropy and heat See Exercises.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
6
A coal-fired power plant produces 900 MW of electric power and operates at a thermal efficiency of 25% (a) What is the input heat rate from the burning coal (b) What is the rate of heat discharge from the plant (c) Water at 15 °C from a nearby river is used to cool the discharged heat. If the cooling water is not to exceed a temperature of 40 °C, how many gallons per minute of the cooling water is required

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
7
A Carnot engine operating between reservoirs at 27 °C and 227 °C does 1500 J of work in each cycle. (a) The change in entropy for the engine for each cycle is (a) negative, (2) zero, (3) positive. Why (b) What is the heat input of the engine

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
8
Only initial and final states are known for irreversible processes on (a) p - V diagrams, (b) p - T diagrams, (c) V - T diagrams, (d) all of the preceding.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
9
An ideal gas initially at temperature T o , pressure p o , and volume V o is compressed to one-half its initial volume. As shown in Fig., process 1 is adiabatic, 2 is isothermal, and 3 is isobaric. Rank the work done on the gas and the final temperatures of the gas, from highest to lowest, for all three processes, and explain how you decided upon your rankings.
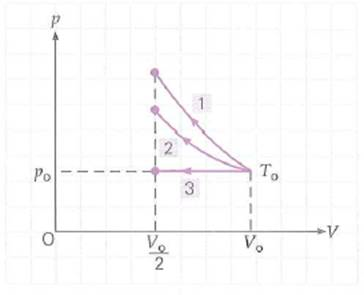
FIGURE Thermodynamic processes See Conceptual Question.

FIGURE Thermodynamic processes See Conceptual Question.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
10
A monatomic ideal gas ( = 1.67) is compressed adiabatically from a pressure of 1.00 × 10 5 Pa and volume of 240 L to a volume of 40.0 L. (a) What is the final pressure of the gas (b) How much work is done on the gas

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
11
If absolute zero were reached, then the Carnot efficiency could be (a) 0%, (b) 50%, (c) 75%, (d) 100%.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
12
Suppose that the system described by the T-S diagram in Fig. is returned to its original state, state 1, by a reversible process depicted by a straight line from state 3 to state 1. (a) The change in entropy of the system for this overall cyclic process is (1) positive, (2) zero, (3) negative. Explain. (b) How much heat is transferred in the cyclic process [ Hint : See Example 12.6.]
![Suppose that the system described by the T-S diagram in Fig. is returned to its original state, state 1, by a reversible process depicted by a straight line from state 3 to state 1. (a) The change in entropy of the system for this overall cyclic process is (1) positive, (2) zero, (3) negative. Explain. (b) How much heat is transferred in the cyclic process [ Hint : See Example 12.6.] FIGURE Entropy and heat See Exercises.](https://d2lvgg3v3hfg70.cloudfront.net/SM7467/11eb75c5_ca1f_5451_adfc_1b13fda86b83_SM7467_00.jpg)
FIGURE Entropy and heat See Exercises.
![Suppose that the system described by the T-S diagram in Fig. is returned to its original state, state 1, by a reversible process depicted by a straight line from state 3 to state 1. (a) The change in entropy of the system for this overall cyclic process is (1) positive, (2) zero, (3) negative. Explain. (b) How much heat is transferred in the cyclic process [ Hint : See Example 12.6.] FIGURE Entropy and heat See Exercises.](https://d2lvgg3v3hfg70.cloudfront.net/SM7467/11eb75c5_ca1f_5451_adfc_1b13fda86b83_SM7467_00.jpg)
FIGURE Entropy and heat See Exercises.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
13
A gasoline engine has a thermal efficiency of 25.0%. If heat is expelled from the engine at a rate of 1.50 × 10 6 J/h, how long does the engine take to perform a task that requires an amount of work of 1.5 × 10 6 J

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
14
The autoignition temperature of a fuel is defined as the temperature at which a fuel-air mixture would self-explode and ignite. Thus, it sets an upper limit on the temperature of the hot reservoir in an automobile engine. The autoignition temperatures for commonly available gasoline and diesel fuel are about 495 °F and 600 °F, respectively. What are the maximum Carnot efficiencies of a gasoline engine and a diesel engine if the cold reservoir temperature is 40 °C

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
15
On a p - V diagram, sketch a cyclic process that consists of an isothermal expansion followed by an isobaric compression, and lastly followed by an isometric process.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
16
Air ideal gas is taken through the reversible processes shown in Fig. (a) Is the overall change in the internal energy of the gas (1) positive, (2) zero, or (3) negative Explain. (b) In terms of state variables p and V , how much work is done by or on the gas, and (c) what is the net heat transfer in the overall process
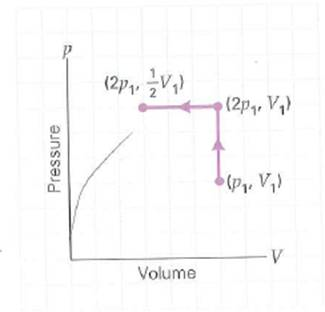
FIGURE A p - V diagram for an ideal gas See Exercise.

FIGURE A p - V diagram for an ideal gas See Exercise.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
17
A thermal pump (a) is rated by thermal efficiency, (b) requires work input, (c) has Q h = Q c , (d) has COP = 1.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
18
The heat output of a thermal pump is greater than the energy used to operate the pump. Does this device violate the first law of thermodynamics

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
19
A 50.0-g ice cube at 0 °C is placed in 500 mL of water at 20 °C. Estimate the change in entropy (after all the ice has melted) (a) for the ice, (b) for the water, and (c) for the ice-water system.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
20
A four-stroke engine runs on the Otto cycle. It delivers 150 hp at 3600 rpm. (a) How many cycles are in 1 min (b) If the thermal efficiency of the engine is 20%, what is the heat input per minute (c) How much heat is wasted (per minute) to the environment

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
21
Because of limitations on materials, the maximum temperature of the superheated steam used in a turbine for the generation of electricity is about 540 °C. (a) If the steam condenser operates at 20 °C, what is the maximum Carnot efficiency of a steam turbine generator (b) The actual efficiency of such generators is about 35% to 40%. What does this range tell you

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
22
An ideal gas goes through a thermodynamic process in which 500 J of work is done on the gas and tire gas loses 300 J of heat. What is the change in internal energy of the gas

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
23
For which type of thermodynamic process is the change in entropy equal to zero: (a) isothermal, (b) isobaric, (c) isometric, or (d) none of the preceding

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
24
Is a living organism an open system or an isolated system Explain.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
25
2.0 kg of ice melts completely into liquid water at 0 °C. (a) The change in entropy of the ice (water) in this process is (1) positive, (2) zero, (3) negative. Explain. (b) What is the change in entropy of the ice (water)

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
26
If an engine does 200 J of net work and exhausts 800 J of heat per cycle, what is its thermal efficiency

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
27
A Carnot engine has an efficiency of 35% and takes in heat from a high-temperature reservoir at 178 °C. What is the Celsius tempera hire of the engine's low-temperature reservoir

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
28
Equation 12.15 shows that the greater the temperature difference between the reservoirs of a heat engine, the greater the engine's Carnot efficiency. Suppose you had the choice of raising the temperature of the high-temperature reservoir by a certain number of kelvins or lowering the temperature of the low-temperature reservoir by the same number of kelvins. (a) To produce the largest increase in efficiency, you should choose (1) to raise the high-temperature reservoir, (2) to lower the low-temperature reservoir, (3) both 1 and 2 produce the same change in efficiency, so it does not matter which you choose. Explain. (b) Prove your answer to part (a) mathematically.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
29
There is no heat flow into or out of the system in an (a) isothermal process, (b) adiabatic process, (c) isobaric process, (d) isometric process.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
30
If ideal gas sample A receives more heat than ideal gas sample B, will sample A experience a higher increase in internal energy Explain.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
31
An ideal gas sample expands isothermally by tripling its volume and doing 5.0 × 10 4 J of work at 40 °C. (a) How many moles of gas arc there in the sample (b) Was heat added to or removed from the sample, and how much

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
32
The maximum efficiency of a heat engine is 1 (or 100%). Can the COP of a thermal pump be greater than 1 Explain.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
33
A gasoline engine has a thermal efficiency of 28%. If the engine absorbs 2000 J of heat per cycle, (a) what is the net work output per cycle (b) How much heat is exhausted per cycle

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
34
A steam engine operates between 100 °C and 20 °C. What is the Carnot efficiency of the ideal engine that operates between these temperatures

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
35
There is a Carnot coefficient of performance (COP C ) for an ideal, or Carnot, refrigerator. (a) Show that this quantity is given by
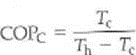
(b) What does this tell you about adjusting the temperatures for the maximum COP of a refrigerator (Can you guess the equation for the COP C for a heat pump )
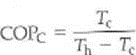
(b) What does this tell you about adjusting the temperatures for the maximum COP of a refrigerator (Can you guess the equation for the COP C for a heat pump )

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
36
In Fig., the plunger of a syringe is pushed in quickly, and the small pieces of paper in the syringe catch fire. Explain this phenomenon using the first law of thermodynamics. (Similarly, in a diesel engine, there are no spark plugs. How can the air-fuel mixture ignite )

FIGURE Syringe fire See Conceptual Question.

FIGURE Syringe fire See Conceptual Question.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
37
A fixed quantity of gas undergoes the reversible changes illustrated in the p - V diagram in Fig. How much work is done in each process
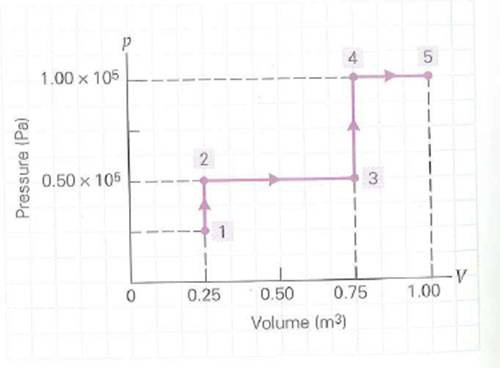
FIGURE A p-V diagram and work See Exercises.

FIGURE A p-V diagram and work See Exercises.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following determines the thermal efficiency of a heat engine: (a) Q c × Q h , (b) Q c / Q h , (c) Q h Q c , or (d) Q h + Q c

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
39
A process involves 1.0 kg of steam condensing to water at 100 °C. (a) The change in entropy of the steam (water) is (1) positive, (2) zero, (3) negative. Why (b) What is the change in entropy of the steam (water)

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
40
A heat engine with a thermal efficiency of 20% does 500 J of net work each cycle. How much heat per cycle is lost to the low-temperature reservoir

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
41
It has been proposed that temperature differences in the ocean could be used to run a heat engine to generate electricity. In tropical regions, the water temperature is about 25 °C at the surface and about 5 °C at very deep depths. (a) What would be the maximum theoretical efficiency of such an engine (b) Would a heat engine with such a low efficiency be practical Explain.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
42
A salesperson tells you that a new refrigerator with a high COP removes 2.6 × 10 3 J (each cycle) from the inside of the refrigerator at 5.0 °C and expels 2.8 × 10 3 J into the 30 °C kitchen, (a) what is the refrigerator's COP (b) Is this scenario possible Justify your answer. (See Exercise.)
Exercise
There is a Carnot coefficient of performance (COP C ) for an ideal, or Carnot, refrigerator. (a) Show that this quantity is given by
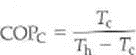
(b) What does this tell you about adjusting the temperatures for the maximum COP of a refrigerator (Can you guess the equation for the COP C for a heat pump )
Exercise
There is a Carnot coefficient of performance (COP C ) for an ideal, or Carnot, refrigerator. (a) Show that this quantity is given by
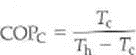
(b) What does this tell you about adjusting the temperatures for the maximum COP of a refrigerator (Can you guess the equation for the COP C for a heat pump )

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
43
While doing 500 J of work, an ideal gas expands adiabatically to 1.5 times its initial volume. (a) Tire temperature of the gas (1) increases, (2) remains the same, (3)decreases. Why (b) What is the change in the internal energy of the gas

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
44
Which one of the following statements is a violation of the second law of thermodynamics: (a) heat flows naturally from hot to cold, (b) heat can be completely converted to mechanical work, (c) the entropy of the universe can never decrease, or (d) it is not possible to construct a perpetual motion engine

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
45
A student tries to cool his dormitory room by opening the refrigerator door. Will that work Explain.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
46
Diesel engines are more efficient than gasoline engines. Which type of engine wold you expect to run hotter Why

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
47
An internal combustion engine with a thermal efficiency of 15.0% absorbs 1.75 × 10 5 J of heat from the hot reservoir. How much heat is lost by the engine in each cycle

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
48
What is the Celsius temperature of the hot reservoir of a Carnot engine that is 32% efficient and has a 20 °C cold reservoir

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
49
An ideal heat pump is equivalent to a Carnot engine running in reverse. (a) Show that the Carnot COP of the heat pump is
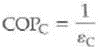
where c is the Carnot efficiency of the heat engine. (b) If a Carnot engine has an efficiency of 40%, what would be the COP C when it runs in reverse as a heat pump (See Exercise.)
Exercise
There is a Carnot coefficient of performance (COP C ) for an ideal, or Carnot, refrigerator. (a) Show that this quantity is given by
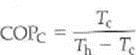
(b) What does this tell you about adjusting the temperatures for the maximum COP of a refrigerator (Can you guess the equation for the COP C for a heat pump )
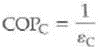
where c is the Carnot efficiency of the heat engine. (b) If a Carnot engine has an efficiency of 40%, what would be the COP C when it runs in reverse as a heat pump (See Exercise.)
Exercise
There is a Carnot coefficient of performance (COP C ) for an ideal, or Carnot, refrigerator. (a) Show that this quantity is given by
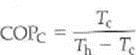
(b) What does this tell you about adjusting the temperatures for the maximum COP of a refrigerator (Can you guess the equation for the COP C for a heat pump )

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
50
If the work done by a system is equal to zero, the process is (a) isothermal, (b) adiabatic, (c) isobaric, (d) isometric.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
51
Heat is converted to mechanical energy in many applications, such as cars. Is this a violation of the second law of thermodynamics Explain.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
52
The temperature of 2.0 mol of ideal gas is increased from 150 °C to 250 °C by two different processes, hi process A, 2500 J of heat is added to the gas; in process B, 3000 J of heat is added. (a) In which case is more work done: (1) process A, (2) process B, or (3) the same amount of work is done Explain. [ Hint : See Eq..] (b) Calculate the change in internal energy and work done for each process.
Equation
![The temperature of 2.0 mol of ideal gas is increased from 150 °C to 250 °C by two different processes, hi process A, 2500 J of heat is added to the gas; in process B, 3000 J of heat is added. (a) In which case is more work done: (1) process A, (2) process B, or (3) the same amount of work is done Explain. [ Hint : See Eq..] (b) Calculate the change in internal energy and work done for each process. Equation](https://d2lvgg3v3hfg70.cloudfront.net/SM7467/11eb75c5_ca00_f72b_adfc_f1c4594c42b7_SM7467_11.jpg)
Equation
![The temperature of 2.0 mol of ideal gas is increased from 150 °C to 250 °C by two different processes, hi process A, 2500 J of heat is added to the gas; in process B, 3000 J of heat is added. (a) In which case is more work done: (1) process A, (2) process B, or (3) the same amount of work is done Explain. [ Hint : See Eq..] (b) Calculate the change in internal energy and work done for each process. Equation](https://d2lvgg3v3hfg70.cloudfront.net/SM7467/11eb75c5_ca00_f72b_adfc_f1c4594c42b7_SM7467_11.jpg)

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
53
During a liquid-to-solid phase change of a substance, its change in entropy is 4.19 × 10 3 J/K. If 1.67 × 10 6 J of heat is removed in the process, what is the freezing point of the substance in degrees Celsius

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
54
The heat output of a particular engine is 7.5 × 10 3 J per cycle, and the net work out is 4.0 × 10 3 J per cycle. (a) The heat input is (1) less than 4.0 × 10 3 J, (2) between 4.0 × 10 3 J and 7.5 × 10 3 J, (3) greater than 7.5 × 10 3 J. Explain. (b) What is the heat input and thermal efficiency of the engine

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
55
An engineer wants to run a heat engine with an efficiency of 40% between a high-temperature reservoir at 300 °C and a low-temperature reservoir. What is the maximum Celsius temperature of the low-temperature reservoir

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
56
A heat engine with a thermal efficiency of 25% is used to hoist 2.5-kg bricks to an elevation of 3.0 m. If the engine expels heat to the environment at a rate of 1.2 × 10 6 J/h, how many bricks can the engine hoist in 2.0 h

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
57
Discuss heat, work, and the change in internal energy of your body when you shovel snow.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
58
Suppose that after the final process in Fig. (see Exercise), the pressure of the gas is decreased isometrically from 1.0 × 10 5 Pa to 0.70 × 10 5 Pa, and then the gas is compressed isobarically from 1.0 m 3 to 0.80 m 3. What is the total work done in all of these processes, including 1 through 5
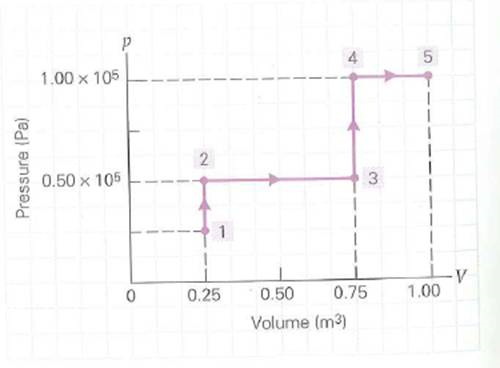
FIGURE A p-V diagram and work See Exercise.
Exercise
A fixed quantity of gas undergoes the reversible changes illustrated in the p - V diagram in Fig. How much work is done in each process

FIGURE A p-V diagram and work See Exercise.
Exercise
A fixed quantity of gas undergoes the reversible changes illustrated in the p - V diagram in Fig. How much work is done in each process

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
59
The Carnot cycle consists of (a) two isobaric and two isothermal processes, (b) two isometric and two adiabatic processes, (c) two adiabatic and two isothermal processes, (d) four arbitrary processes that return the system to its initial state.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
60
If you have the choice of running your heat engine between either of the following two sets of temperatures for the cold and hot reservoirs, which would you choose, and why: between 100 °C and 300 °C, or between 50 °C and 250 °C

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
61
A gasoline engine burns fuel that releases 3.3 × 10 8 J of heat per hour. (a) What is the energy input during a 2.0-h period (b) If the engine delivers 25 kW of power during this time, what is its thermal efficiency

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
62
A Carnot engine with an efficiency of 40% operates with a low-temperature reservoir at 40 °C and exhausts 1200 J of heat each cycle. What are (a) the heat input per cycle and (b) the Celsius temperature of the high-temperature reservoir

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
63
When cruising at 75 mi/h on a highway, a car's engine develops 45 hp. If tills engine has a thermodynamic efficiency of 25% and 1 gal of gasoline has an energy content of 1.3 × 10 8 J, what is the fuel efficiency (in miles per gallon) of this car

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
64
Explain why the process shown in Fig. 12.1b is not that for an ideal gas at constant temperature.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
65
An ideal gas expands from 1.0 m 3 to 3.0 m 3 at atmospheric pressure while absorbing 5.0 × 10 5 J of heat in the process. (a) The temperature of the system (1) increases, (2) stays the same, (3) decreases. Explain. (b) What is the change in internal energy of the system

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
66
An ideal gas is compressed isothermally. The change in entropy of the gas for this process is (a) positive, negative, (c) zero, (d) none of the preceding.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
67
What happens to the pressure and internal energy of a cyclic heat engine after a complete cycle

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
68
In an isothermal expansion at 27 °C, an ideal gas does 60 J of work. What is the change in entropy of the gas

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
69
A steam engine is to have its thermal efficiency improved from 8.00% to 10.0% while continuing to produce 4500 J of useful work each cycle. (a) Does the ratio of the heat output to heat input (1) increase, (2) remain the same, or (3) decrease Why (b) What is the change in Q c / Q h in this example

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
70
A Carnot engine takes 2.7 × 10 4 J of heat per cycle from a high-temperature reservoir at 320 °C and exhausts some of it to a low-temperature reservoir at 120 °C How much net work is done by the engine per cycle

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
71
A gram of water (volume of 1.00 cm 3 ) at 100 °C is converted to 1.67 × 10 3 cm 3 of steam at atmospheric pressure. What is the change in the internal energy of the water (steam)

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
72
While playing in a tennis match, you lost 6.5 × 10 5 J of heat, and your internal energy also decreased by 1.2 × 10 6 J. How much work did you do in the match

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
73
According to the first law of thermodynamics, if work is done on a system, then (a) the internal energy of the system must change, (b) heat must be transferred from the system, (c) the internal energy of the system may change and/or heat may be transferred from the system, (d) heat must be transferred to the system.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
74
Does the entropy of each of the following objects increase or decrease (a) Ice as it melts; (b) water vapor as it condenses; (c) water as it is heated on a stove; (d) food as it is cooled in a refrigerator.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
75
One hand red moles of a monatomic gas is compressed as shown on the p - V diagram in Fig. (a) Is the work done by the gas (1) positive, (2) zero, or (3) negative Why (b) What is the work done by the gas (c) What is the change in temperature of the gas (d) What is the change in internal energy of the gas (e) How much heat is involved in the process
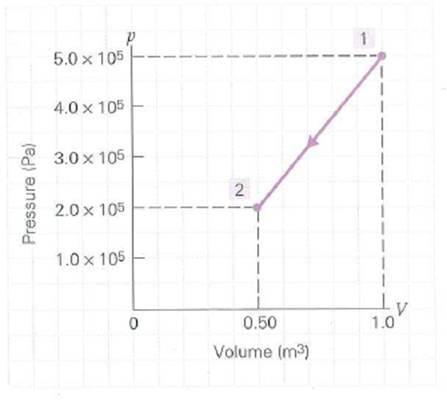
FIGURE A variable p-V process and work See Exercise.

FIGURE A variable p-V process and work See Exercise.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
76
Carnot engine A operates at a higher hot reservoir temperature than Carnot engine B. Will engine A necessarily have a higher Carnot efficiency Explain.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
77
An engineer redesigns a heat engine and improves its thermal efficiency from 20% to 25%. (a) Does the ratio of the heat input to heat output (1) increase, (2) remain the same, or (3) decrease Explain. (b) What is the engine's change in Q h / Q c

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
78
A Carnot engine takes in heat from a reservoir at 350 °C and has an efficiency of 35%. The exhaust temperature is not changed and the efficiency is increased to 40%. (a) The temperature of the hot reservoir is (1) lower than (2) equal to, (3) higher than 350 °C. Explain. (b) What is the new Celsius temperature of the hot reservoir

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
79
In a highly competitive game, a basketball player can produce 300 W of power. Assuming the efficiency of the player's "engine" is 15% and heat dissipates primarily through the evaporation of perspiration, what mass of perspiration is evaporated per hour

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
80
On a p - V diagram, a reversible process is a process (a) whose path is known, (b) whose path is unknown, (c) for which the intermediate steps are nonequilibrium states, (d)none of the preceding.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck



